Published: January 2024; Last updated: April 2024
Summary
What do they do?
This review focuses only on Malaria Consortium’s work on seasonal malaria chemoprevention (SMC) in sub-Saharan Africa. SMC involves giving children monthly courses of antimalarial medicines in locations where malaria is highly seasonal (i.e., a high proportion of cases occur in a relatively short period each year) (more). Malaria Consortium provides funding and operational support to deliver SMC campaigns for preschool-age children (more).
We recommend Malaria Consortium’s SMC program because of its:
- Focus on a program with strong evidence of effectiveness at reducing child mortality. (More in our separate report on SMC)
- Strong track record as a leader and innovator in the SMC field. (More)
- Involvement in the SMC programs it supports at all stages, from planning to delivery. We would guess that this gives it strong oversight and a greater ability to address problems when they occur. (More)
- Strong processes for tracking what proportion of children received SMC in its campaigns. (More)
- Very positive feedback from national malaria programs and other SMC stakeholders. (More)
- Transparency – Malaria Consortium shares significant information about its work with us and we are able to closely follow and understand its work. (More)
We do not currently have any significant concerns about Malaria Consortium as an organization. We do have a number of uncertainties about SMC, including the level of malaria mortality in areas where GiveWell supports campaigns, and whether SMC campaigns would be funded by other malaria funders in GiveWell’s absence. We discuss these in our separate report on SMC.
These assessments are based on the following components:
What do you get for your dollar? We believe Malaria Consortium’s SMC program is one of the most cost-effective programs that donors can support. As of December 2023, we estimate that it costs approximately $2,000 to $7,000 (depending on the location) to avert a death in areas where GiveWell supports SMC. SMC is cost-effective because Malaria Consortium’s program reaches a high proportion of targeted children (who would not otherwise receive SMC), there is strong evidence that SMC reduces malaria, and we think SMC probably provides substantial additional benefits like increased income in later life. See our separate report on SMC for more detail.
What information has Malaria Consortium shared on its program? We ask organizations that we fund to share monitoring data and other detailed information on their programs. We use the data as inputs in our cost-effectiveness analysis, and its quality and reliability also inform our overall assessment of the program. For Malaria Consortium, this includes:
- Post-campaign coverage surveys to understand how many children received SMC. These surveys have generally found high proportions of children reached (approximately 80% - 90% overall, varying by country) (more).
- Information on costs incurred on the campaigns it supports, although these are not comprehensive in all countries (more).
We think the information we have seen is reliable and high-quality overall. It gives us confidence that Malaria Consortium is delivering SMC at high quality, and that its programs are reaching a high proportion of children at low cost.
What is GiveWell’s qualitative assessment of Malaria Consortium? We make qualitative assessments of our top charities alongside our cost-effectiveness analyses to inform our grantmaking. Overall, our assessment of Malaria Consortium is highly positive, even compared to our other top charities. We believe it stands out on a number of factors including (more):
- Role in the field: Malaria Consortium is a leading organization in the SMC field and has played a major role in its expansion to new locations.
- Technical expertise: Malaria Consortium has dedicated research and technical staff, providing capacity to conduct large-scale studies (like randomized controlled trials of SMC in Mozambique and Uganda), and high-quality monitoring.
- Quality of information shared: The information we have received from Malaria Consortium has been detailed and easy to interpret. This gives us confidence that we understand its SMC program in detail. Malaria Consortium has also told us about challenges its program faces, which increases our confidence in its transparency.
1. What do they do?
1.1 What is Malaria Consortium?
Malaria Consortium works on preventing, controlling, treating, and eliminating malaria and other communicable diseases.1 It was established in 2003 and currently works in thirteen countries across Africa and Southeast Asia.2
This page focuses exclusively on its seasonal malaria chemoprevention (SMC) programs, which distribute antimalarial drugs to young children. As of December 2023, GiveWell has also funded Malaria Consortium to deliver insecticide-treated net (ITN) campaigns to control malaria in two states in Nigeria (details on a separate page).3
1.2 What is seasonal malaria chemoprevention (SMC)?
Seasonal malaria chemoprevention (SMC) involves giving children monthly courses of antimalarial medicines in locations where malaria is highly seasonal (i.e., a high proportion of cases occur in a relatively short period each year). SMC is delivered to all children in a given location4 (exceptions in footnote).5 The antimalarial medicines used are sulfadoxine-pyrimethamine (SP) and amodiaquine (AQ).6
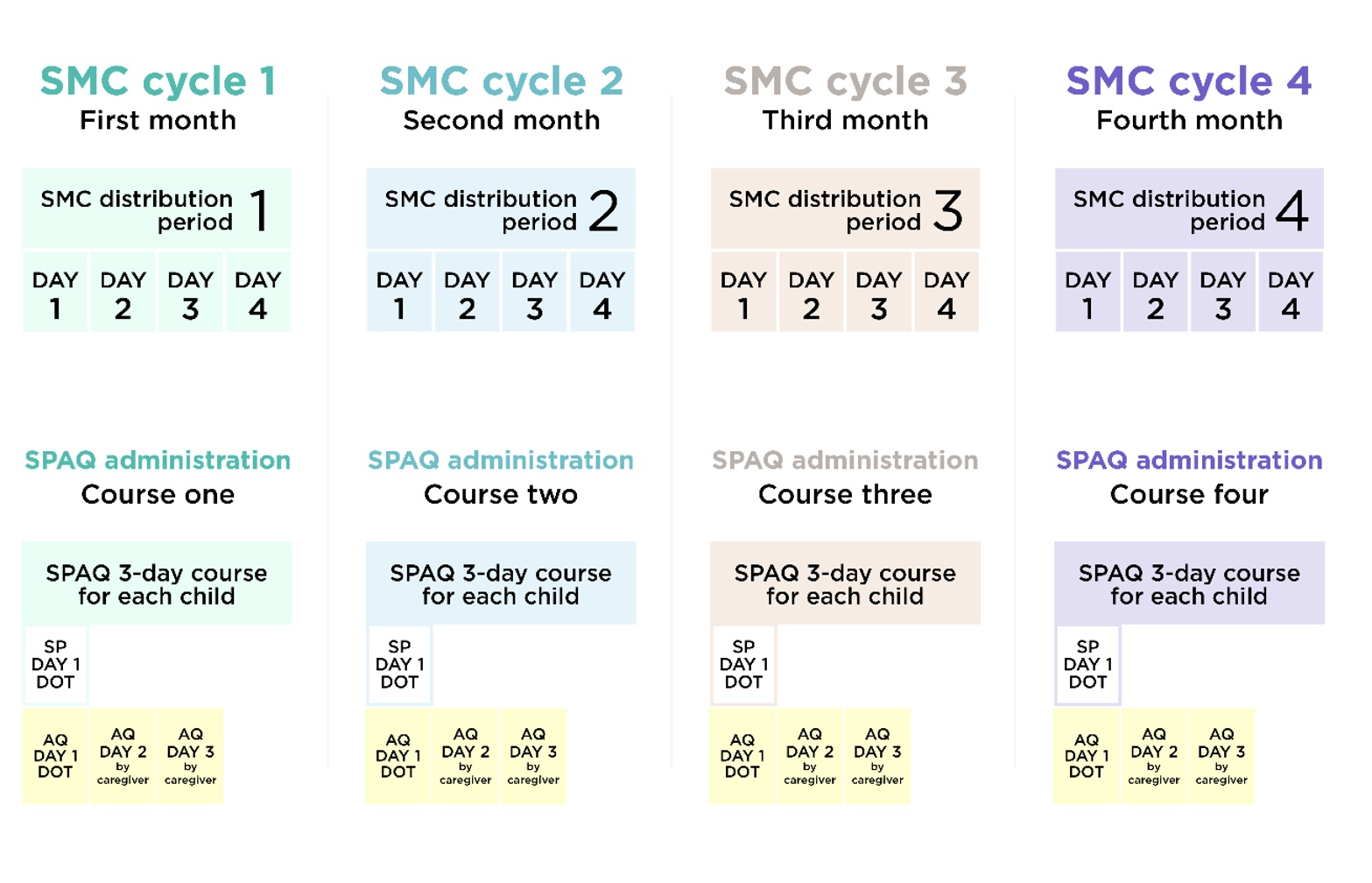
SMC is delivered in cycles at 28-day intervals. Each cycle involves giving children four doses of medicine over three days. Trained community distributors give children the first day’s doses (SP and AQ) directly, and give the doses for days two and three (AQ only) to the child’s caregiver to administer themselves.7 Each annual season of SMC (usually four or five cycles depending on the location) is referred to as a “round.”8
The World Health Organization (WHO) has recommended SMC for deployment since 2012.9 The original WHO recommendation was for up to four monthly cycles for children aged 3–59 months in areas where more than 60% of the annual incidence of malaria occurs within four months and where resistance to the medicines used in SMC was low.10
SMC has scaled up rapidly since it was first recommended by the WHO. An estimated 2.6 million children were reached with SMC in 2014, rising to approximately 49 million in 2022.11
In recent years, some countries began to expand their use of SMC beyond the original WHO recommendation. These changes included delivering five cycles rather than four in some locations (for example, in Nigeria and Burkina Faso beginning in 2021),12 and pilots of SMC in Mozambique and Uganda in 2020/21.13 In 2022, the WHO updated its policy recommendation for SMC in line with these developments. The key changes included:14
- Removing geographic restrictions on the use of SMC
- Allowing for variability in the number of cycles delivered
- More flexibility on the age range for targeted children (although in most countries, the WHO states that children under five will still be highest priority)15
Following these changes, SMC—which has historically only been delivered at large scale in countries in the Sahel region of West Africa, where drug resistance is low—we expect SMC to expand more rapidly in other areas with highly seasonal malaria transmission, contingent on availability of funding.
1.3 How Malaria Consortium’s SMC program works
Overview
SMC campaigns are implemented under the leadership of national malaria programs. In the locations where GiveWell funds Malaria Consortium to support SMC, it provides the funding to deliver SMC campaigns,16 trains distributors to deliver the campaigns, and provides technical and operational support to governments on all aspects of campaign delivery (more). It also conducts monitoring after campaigns to understand what proportion of children were reached (more).
Malaria Consortium estimates that its SMC program targeted 24 million children with SMC in seven countries in 2022.17 This accounts for approximately half of the total number of children (49 million) reached with SMC in that year.18 Of these, approximately two-thirds (~15 million children) were targeted with exclusively philanthropic funding19 (which primarily comprises funding directed by GiveWell). The remainder were targeted through funding from the Global Fund to Fight AIDS, Tuberculosis and Malaria (Global Fund), or through co-funding arrangements.20 The seven countries where Malaria Consortium supported delivery of SMC are highlighted in the map below.

Countries with Malaria Consortium-supported SMC programs in 2022 are highlighted in blue.21
Malaria Consortium SMC implementation methods
Malaria Consortium supports training of health facility workers and community distributors (CDs) to deliver SMC primarily by going door-to-door.22 Some CDs are community health workers (CHWs), who are people in the community who support basic delivery of health interventions. The majority of CDs are recruited and trained to only deliver SMC and do not provide any other community health services.23 As of 2016-2017, Malaria Consortium told us that CDs are typically paid about $5 to $7 per day for programs such as SMC, though this amount varies by country.24 Malaria Consortium also supports training for supervisors for the program.25
As discussed above, national governments have some flexibility about how many cycles of SMC to deliver per year in each location. Malaria Consortium estimates that, in 2022, just under ⅔ (62%) of children supported by Malaria Consortium’s philanthropically funded SMC program lived in areas where four SMC cycles were implemented. The remaining 38% received five cycles.26 The areas receiving five cycles include some parts of Burkina Faso and Nigeria (whose peak malaria transmission season is longer than in other SMC-eligible locations)27 and all SMC-implementing areas supported by Malaria Consortium in South Sudan and Uganda.28 Each cycle involves giving preschool-age29 children antimalarial drugs over a three-day period30 and lasts 28 days, at which point a new cycle starts.31 For each cycle, Malaria Consortium instructs CDs to:32
- Determine whether the child is eligible for SMC and give the age-appropriate dose.33
- Refer all acutely sick children and children with fever to the health facility or a qualified community health worker for evaluation and testing for malaria.34
- Directly observe the child swallowing the first dose of dispersible SP+AQ, and then re-dose if the child vomits or spits out all of the medicine within 30 minutes of taking the first dose of the medication.35
- Give the child's caregiver 2 tablets of AQ and explain how to give the doses over the following two days.36
- Record all doses provided on a tally sheet and mark the wall of the household or compound as visited.37
- Advise the child's caregivers to mark a card to record that they've given the other two doses,38 to give the medication again if the child vomits (and to visit the health facility to request replacement doses if this happens), and to take the child to the health facility if they get a fever or are very sick.39
- Provide health promotion and malaria prevention messages, including explaining the purpose and benefit of SMC and the importance of children and pregnant women sleeping inside a bed net each night.40
This is a diagram of the delivery schedule (for a four-cycle distribution):41
Malaria Consortium's role in SMC programs
Malaria Consortium's SMC work varies by country, but in general aims to support countries' national malaria programs in implementing high-quality SMC campaigns, including the following activities:42
- Funding distributions. In locations where it receives funding from GiveWell,43 Malaria Consortium funds activities including in-country storage and transportation of drugs and payments to front-line distributors to compensate them for the time they spend on the program.
- Planning and enumeration. About five months before the SMC round, Malaria Consortium collaborates with governments and other partners to begin planning for the round and budgeting based on enumeration of the target population, personnel, and commodities costs.44
- Procurement and supply management. Malaria Consortium works with countries to determine the quantity of drugs needed, procures the drugs and other commodities45 needed for SMC delivery, and manages international shipping.46 Malaria Consortium typically places orders for SMC drugs about a year before the start of the SMC round.47
- Training and supervision. Malaria Consortium supports the training of CDs to identify eligible children, refer sick children to care, and record SPAQ administration.48 Program supervisors oversee CDs to provide mentoring and constructive feedback on implementation.49
- Community engagement. Malaria Consortium supports activities to promote community engagement and social and behavior change before and during SMC campaigns.50
- SMC research. Malaria Consortium also conducts operational research to assess the feasibility and impact of modifying the procedures described above, as well as implementation research and impact analyses.51
- Financial management and oversight, including disbursing funds to local organizations, Ministries of Health, and/or CDs, collecting and validating receipts, and preparing financial reports.
- Advocacy and fundraising with governments, international, multinational, and bilateral organizations, donors, SMC working groups, researchers, and civil society.
Malaria Consortium also conducts monitoring surveys after its campaigns to estimate what proportion of children received SMC. These surveys are discussed in detail below.
1.4 What SMC programs has Malaria Consortium delivered?
Since 2017, Malaria Consortium has been using funding received as a result of GiveWell's recommendation (which we refer to as "GiveWell-directed funds" and Malaria Consortium refers to as “philanthropic funding”) to support SMC programs in several countries. In 2022, this funding accounted for approximately two-thirds of the approximately 24 million children that Malaria Consortium targeted with SMC in 2022, with funding from the Global Fund accounting for most of the remaining children.52
Over time, the total number of children targeted by GiveWell-directed funds (either in full or in part) has increased from about 660,000 in 2017 to about 16.1 million in 2022.53 In 2022, Malaria Consortium used GiveWell-directed funds or co-funding to target approximately 2.1 million children in Burkina Faso, approximately 1.2 million children in Chad, approximately 10.7 million children in Nigeria, and approximately 500,000 children in Togo.54
Since 2020-2021, Malaria Consortium has also started to expand its SMC program to locations outside the Sahel region of West Africa:
- Mozambique: Malaria Consortium supports SMC in Nampula province in Mozambique. Using GiveWell funding, Malaria Consortium piloted SMC in two districts in Nampula in the 2020-2021 and 2021-2022 SMC seasons.55 Malaria Consortium also conducted a two-year implementation study involving several research components, including a randomized controlled trial (RCT) of SMC's effectiveness in Nampula. GiveWell co-funded this research with the Bill & Melinda Gates Foundation (BMGF). In February 2022 (before seeing the RCT results), GiveWell made a grant to Malaria Consortium to scale up and deliver SMC to all of Nampula province during the 2022-2023 and 2023-2024 SMC seasons.56
- Uganda: Malaria Consortium supports SMC in Karamoja region, where the story has been similar to the story in Nampula. GiveWell funded Malaria Consortium to pilot SMC in Karamoja in 2021 and 2022 and co-funded a similar two-year implementation study, (including an RCT), with BMGF.57 In 2023, Malaria Consortium delivered SMC to the whole Karamoja region using philanthropic and Global Fund funding.
- South Sudan: Beginning in 2022, Malaria Consortium also conducted an implementation study in South Sudan, targeting approximately 20,000 children in one county.58
We have also seen information from two previous SMC projects that Malaria Consortium has supported:
- Pilot and scale-up of SMC in northern Nigeria: BMGF provided about $1.7 million to Malaria Consortium to do operational research on the best way to deliver SMC at scale in Katsina state in northern Nigeria, and then to implement its chosen delivery system and assess its efficiency and impact.59 Malaria Consortium told us that it trained over 3,600 CDs and nearly 200 health workers to provide about 1.6 million courses of SMC to roughly 350,000 children who lived in 4 "local government areas" (LGAs) in northern Nigeria in 2012-2014.60
- ACCESS-SMC:61 Unitaid awarded up to $67 million to Malaria Consortium to lead a project called ACCESS-SMC to reach up to 7 million children per year in seven countries in the Sahel region of Africa in 2015-2017.62 The project, which Malaria Consortium described in 2014 as being the "largest-yet global programme" for SMC, concluded in February 2018.63 ACCESS-SMC was led by Malaria Consortium, with Catholic Relief Services as the "primary sub-grantee" and support from many other organizations, including impact evaluation from the London School of Hygiene & Tropical Medicine (LSHTM).64 Malaria Consortium told us that its role in ACCESS-SMC included leading implementation of SMC in three of the seven ACCESS-SMC countries (Burkina Faso, Chad, and Nigeria), overseeing budgets and planning for all ACCESS-SMC activities, and overseeing research (including methodology and presentation).65 Our impression is that ACCESS-SMC paid for almost all aspects of program implementation and monitoring, including medicines and supplies, per diems for CDs, training for CDs, trainers, supervisors, and health facility workers, and research.66 ACCESS-SMC generated evidence for the subsequent scale-up of SMC by providing evidence of impact at scale under programmatic conditions.67
1.5 Malaria Consortium’s spending on SMC programs
Between January and December 2022, Malaria Consortium spent $69.7m in GiveWell-directed funds:68
| Category | Spending ($ US) |
% of total (2022) |
|---|---|---|
| SMC medicines, freight, and procurement | $24.8m | 36% |
| SMC implementation (inc. planning, training, administration of medicines, community engagement, and monitoring and evaluation, including coverage surveys) | $26.7m | 38% |
| Staff and travel costs69 | $7.3m | 10% |
| Equipment | $0.6m | 1% |
| Research, communications, and advocacy70 | $1.8m | 2% |
| Overheads | $8.7m | 12% |
| Total | $69.7m | 100% |
Of this, Malaria Consortium spent $45m (64%) in Nigeria, $9.0m (13%) in Burkina Faso, $5.7m (8%) in Mozambique, $5.4m (8%) in Chad, $2.2m (3%) in Uganda, $1.5m (2%) in Togo, and $1.1m (2%) in South Sudan, with above-country spending allocated proportionally across countries.71
2. Monitoring and information sharing
2.1 Overview
GiveWell asks organizations that we fund to share detailed information on their programs. The aim of reviewing this information is to assess how much the program costs, whether the program is being conducted to a high quality, and whether it is reaching recipients as intended. We use data from these reviews as inputs in our cost-effectiveness analyses, and the quality and reliability of the information we’ve seen also inform our qualitative assessment of the program.
Malaria Consortium has shared detailed information that we use to evaluate its SMC program. The information that we rely on most is:72
- Monitoring data from previous campaigns, to estimate the proportion of children reached (more).
- Information on costs incurred on previous Malaria Consortium-supported campaigns (both Malaria Consortium’s costs and other actors’) (more).
- A descriptive annual report on its program activities (more).
Overall, the information we have seen provides gives us confidence that:
- Malaria Consortium-supported campaigns are reaching a high proportion of children. Our review of monitoring data finds that the overall percentage of targeted children reached in Malaria Consortium-supported campaigns between 2017 and 2021 was 78% in Nigeria, 85% in Chad, 87% in Togo, and 92% in Burkina Faso73 (more). We are relatively confident in the quality of Malaria Consortium’s monitoring (more).
- Malaria Consortium delivers SMC at low cost. Based on data on the proportion of children reached and the cost information that Malaria Consortium shares, we estimate that it costs around $1.50 in total to deliver each cycle of SMC to a child in Malaria Consortium-supported campaigns (more).
Although we think the information we have seen from Malaria Consortium and use in our analysis is relatively comprehensive and high-quality, we think its main shortcomings are:
- Lack of information on other actors’ spending in Togo. We use a weighted average figure for other countries ($1.50) in place of a cost-per-cycle estimate in Togo, because until recently we did not have information on all co-funders’ SMC spending. We therefore expect our estimate in Togo to be less accurate than in other locations (more).
- Concerns about some aspects of Malaria Consortium’s post-campaign monitoring. The main points are:
- Discrepancies between post-round and post-cycle surveys for some campaigns (more).
- Reliance on self-reported monitoring data (caregivers reporting whether their child received SMC). (More)
- No auditing procedure in most of the surveys we have reviewed (more).
- Uncertainty about the target population estimates used in our cost analysis (more).
2.2 Monitoring and evaluation
Overview
Malaria Consortium conducts coverage surveys to determine what proportion of the target population of children received SMC in previous campaigns. We use this data to understand whether the program is reaching children as intended, and as part of our estimates of the costs to deliver each cycle of SMC (discussed in our separate report on SMC).
Overall, we believe that results from Malaria Consortium’s coverage surveys provide relatively strong evidence that a high proportion of the target population has been reached with SMC in previous campaigns. Overall coverage (the % of target children receiving SMC) from post-round surveys74 has been 78% in Nigeria, 85% in Chad, 87% in Togo, and 92% in Burkina Faso.75
Our main open questions and reservations about Malaria Consortium’s coverage surveys are:
- Discrepancies between post-round and post-cycle surveys for some campaigns. Malaria Consortium conducts two types of coverage survey: one survey after each monthly cycle of SMC, and a separate survey at the end of the whole SMC round (four or five months, depending on the location). In some campaigns, there have been significant discrepancies in the coverage measured between these surveys.76 To account for this, we use results from the post-round surveys (which we think are likely to be more accurate) in our cost-per-cycle estimates, but these discrepancies reduce our overall confidence in the accuracy of the coverage data (more).
- Reliance on self-reported monitoring data. In Malaria Consortium’s surveys, SMC coverage is measured by asking caregivers to self-report whether their child received SMC. We would have more confidence in a survey that validated these responses against a more objective measure. (more)
- No auditing procedure in most of the surveys we have reviewed. In 2021, Malaria Consortium introduced an audit procedure in some of its monitoring surveys, but most of the results we have seen have not been audited. We would have more confidence in survey data that was fully audited (more).
- Uncertainty about target population data. We’re uncertain about the accuracy of the target population estimates for 3-59 month old children used in our analysis. These may be inaccurate for various reasons (e.g., differential population growth at the local level, migration, or internal displacement) (more).77
Comprehensiveness
See this spreadsheet for all coverage survey results we have seen from Malaria Consortium's SMC programs. In short, we have seen results from all large-scale SMC programs78 that Malaria Consortium supported with GiveWell-directed funds through 2021. This includes results from 2017-2021 in Burkina Faso, Chad, and Nigeria, from 2020-2021 in Togo, and from 2021 in Uganda and Mozambique.79
We have also seen results from 2015-2016 in the seven countries that Malaria Consortium supported through the ACCESS-SMC project.80 We thus believe that we have seen a thorough picture of the impact of Malaria Consortium's SMC program.
We focus our review on results from 2017 onward in Burkina Faso, Chad, and Nigeria and from 2020 onward in Togo, as we believe they are more likely to be indicative of what we can expect from future SMC rounds in those countries. We do not focus on results from Mozambique and Uganda because Malaria Consortium was operating at a small scale and doing implementation research in these countries in 2021, so we don't expect results to provide a good indication of the coverage that it might achieve in future program years.81
Methodology
Since 2017, Malaria Consortium has conducted two types of coverage surveys. After all but the final cycle in the SMC round, it conducts a post-cycle coverage survey to measure coverage in the previous cycle only.82 After the final cycle in the SMC round, it conducts a post-round coverage survey to measure coverage across the full round. Both post-cycle and post-round surveys involve household interviews with caregivers of SMC-eligible children. Full details of the methodology used in the surveys we have reviewed are in the "Methods" sheets of this spreadsheet.
Below, we summarize Malaria Consortium's general post-cycle and post-round coverage survey methodology and discuss methodological strengths and weaknesses. Overall, we believe that both types of surveys are designed to measure key indicators of the success of SMC campaigns and to achieve samples that are generally representative of target populations. However, we are concerned that the self-reported nature of responses and data collectors' involvement in the later stages of respondent selection may produce bias in results. In general, we have also been uncertain about the quality of survey implementation due to the lack of a procedure to audit data collectors' work. In 2021, auditing measures were introduced into post-round and post-cycle surveys in Nigeria (and post-round surveys in Mozambique and Uganda).83 We see this as a methodological strength, both because such a procedure may encourage accurate data collection and because it provides a check on the accuracy of results. Results from the auditing of post-round surveys in Nigeria suggest that in the vast majority of cases, data collection procedures were followed correctly.84
Key features of Malaria Consortium’s survey methodology include:
- Respondent selection: Post-cycle surveys employ lot quality assurance sampling85
in which the program area is subdivided into smaller units, generally of approximately equal population size, and a small number of households is randomly selected from each unit.86
While this approach is primarily designed to assess whether each unit met a target coverage level, data from all units can be aggregated to calculate coverage across the program area. Because all units are sampled and are of approximately equal population size, we expect this selection protocol to result in a sample that is generally representative of the target population.87
Post-round surveys employ multi-stage cluster sampling of households in the program area, with sampling units above the household level generally selected randomly with probability proportional to size.88
We expect this selection protocol to result in a sample that is generally representative of the target population.
For both types of surveys, data collectors are instructed to randomly select households to survey. In some surveys we have reviewed, data collectors were instructed to use numbered household lists from which they selected households using randomly generated numbers. In other surveys, data collectors were instructed to spin a bottle at a central point in the community to choose a direction along which they then selected a predetermined number of households at a predetermined interval.89 This latter method may lead households closer to the center of a community to be overrepresented in the sample. We are unsure of how this might bias results, though it seems plausible that households on the outskirts of a community may have been less likely to be reached by SMC, and thus that results would be biased upward.
Next, data collectors enter all children aged 3-59 months (the eligible age range for SMC) in the selected household into survey software. The survey software then randomly selects a child, and data collectors ask caregivers questions about that child.90
We see it as a methodological strength that child selection is randomized by survey software. However, we see data collectors’ involvement in the later stages of respondent selection as a potential concern. Data collectors may apply selection procedures incorrectly, either unintentionally or intentionally. If, for example, they purposefully select households that are easier to reach, this would be a potential source of upward bias, as households that are easier to reach may also have been more likely to be reached by SMC. We note that we have seen no evidence that data collectors intentionally applied selection procedures incorrectly and note it only as a possibility.
If a selected household is unavailable or refuses to participate in the survey, data collectors are instructed to move to the next household according to the sampling procedure. In the 2019-21 post-round surveys, 98% or higher of the targeted number of households were interviewed.91 This could mean that non-response rates (i.e., households randomly selected to be interviewed not being interviewed) were low. However, because we do not know how often replacement households were used, it is also possible that a high proportion of interviewed households were replacement households, so these survey completion rates only slightly increase our confidence in the accuracy of results from these surveys. The post-cycle survey completion rates for 2020-2021 from Burkina Faso, Chad, Nigeria, and Togo were 90% or higher, with the exception of Plateau State in Nigeria in 2021.92
- Survey design: Malaria Consortium has developed post-cycle and post-round questionnaires,93
which are adapted for use in each country. Questionnaires are translated from English into French in Burkina Faso, Chad, and Togo, and into Portuguese in Mozambique.94
These questionnaires instruct data collectors to ask caregivers questions about whether their child received SPAQ during the previous cycle (in the case of post-cycle surveys) or during all cycles of the round (in the case of post-round surveys). Both questionnaires ask about SPAQ provided by a CD on the first day of each cycle and about AQ provided by the caregiver on the second and third days of each cycle. They also ask questions about the quality of program delivery. Data collectors translate questions from English, French, or Portuguese into local languages during household interviews,95
which may lead to inconsistencies in translation and reduce the accuracy of results.
A potential source of bias in Malaria Consortium's coverage surveys is their heavy reliance on self-reported responses. Post-round responses are at high risk of recall bias, as they report on up to 12 or 15 doses,96 the first of which would have occurred at least three or four months prior.97 Post-cycle responses are at lower risk of recall bias, as they ask only about three doses and are conducted within two weeks to a month of those doses.98 Self-reported responses are also at risk of social desirability bias that could lead caregivers to overreport SMC administration if they believe that this is the preferred response of data collectors. We expect responses about caregivers' own administration on the second and third days of each cycle to be at greater risk of this type of bias, as they may feel pressure to overreport their own adherence to program guidance. We account for this in our cost-effectiveness analysis with a separate adjustment (details in footnote).99
We would have more confidence in a survey that tested the reliability of self-reported responses against some objective measure. In order to verify caregiver responses, data collectors are instructed to review children's SMC record cards and drug blister packs, if available.100 However, retention of these items has generally been low, leading Malaria Consortium to place low weight on them as indicators of coverage.101
- Survey implementation: In general, Malaria Consortium contracts with local research organizations that recruit data collectors and oversee survey implementation.102
Individuals involved in the surveys generally were not involved in SMC delivery, which suggests that they are unlikely to have a personal interest in survey outcomes.103
Malaria Consortium has reported a few cases in which surveys conducted by local research organizations were of low quality.104
This may have impacted the accuracy of results, but we are uncertain about the magnitude or direction of this impact.
Malaria Consortium's coverage surveys have not systematically incorporated an auditing procedure to assess the accuracy of data collectors' work. In 2021, auditing measures were introduced into post-round and post-cycle surveys in Nigeria (and post-round surveys in Mozambique and Uganda).105 We see this as a methodological strength, both because such a procedure may encourage accurate data collection and because it provides a check on the accuracy of results. We have not seen results from the audits of Nigeria post-cycle and Mozambique and Uganda post-round surveys. We have seen results from the audits of post-round surveys in Nigeria in 2021, for which several methods were in place to check enumerators' entries: supervisors tracked enumerators' GPS locations to ensure that they visited the correct communities, listened to a portion of audio recordings taken of interviews, and re-surveyed a portion of households to check that interviews had been conducted according to survey protocol. The vast majority of supervisor re-surveys uncovered no issues, which increases our confidence that data collection procedures were usually followed correctly.106 In addition, we have seen data from repeat interviews conducted in Nigeria in 2017.107
- Data capture: Data is collected electronically in both post-cycle and post-round coverage surveys and uploaded to a remote server at the end of each day of data collection.108 One concern we have about coverage surveys in general is that data may be lost after being collected. As mentioned above, from 2019-2021, data was collected and uploaded from 98% or higher of the number of households that were targeted to be interviewed in post-round surveys and from 90% or higher of households targeted in post-cycle surveys in 2020-2021. This leads us to believe that it is unlikely that substantial data loss occurred after collection.
Detailed results
We believe that results from Malaria Consortium's coverage surveys provide strong evidence that a high proportion of the target population was reached with SMC in past rounds. We use these coverage estimates, along with data on program spending, in our estimates of the cost per cycle of SMC delivered.
See this spreadsheet for all results we have seen from Malaria Consortium's SMC programs. Results weighted by target population from post-round surveys in 2017-2021 show that across those years, for children targeted to receive four cycles of SMC:109
- 95% were covered by SMC for at least one monthly cycle
- 88% for at least two cycles
- 77% for at least three cycles, and
- 65% for all four cycles.
In the most recent program years, post-round surveys measured average coverage across cycles at 92% in Burkina Faso (2018-2020), 85% in Chad (2018-2021), 78% in Nigeria (2018-2021), and 87% in Togo (2020-2021).110
For coverage surveys in 2017-2021, we have compared results from post-cycle and post-round surveys. After converting the two sets of coverage estimates into a measure of total person-months of coverage for comparison, we find a difference of 2% between them for 2017, 13% for 2018, 24% for 2019, 9% for 2020, and 6% for 2021.111 We find it concerning that this difference is high in some years. We don't know why, overall, it increased from 2017-2019 and then decreased in 2020 and 2021, but we have attempted to understand the main drivers of this difference for each year. In general, the difference has been driven by results from Nigeria, with results from Chad also contributing to the difference in 2019.112 From 2018 to 2021, post-cycle results found higher person-months of coverage than post-round results.113 Differences in sampling protocol and questionnaire design between the two types of surveys may inevitably lead to some difference in results. However, the fact that post-cycle and post-round results were similar in Burkina Faso in 2017-2020, in Chad in 2017-2018 and 2020-2021, and in Nigeria in 2017 leads us to believe that larger discrepancies cannot be explained merely by methodological differences and likely result from biases present in the surveys in Chad in 2019 and in Nigeria in 2018-2021. In cases where the discrepancy is large, we believe that the post-round results are a better indication of actual coverage. Our main reasons (further details in footnote)114 are:
- We have previously heard from Malaria Consortium that some researchers for some post-cycle surveys (in Nigeria in 2018) were recruited from among state-level health authority staff. We would generally expect that fully independent surveys will be more accurate.
- Post-cycle surveys have generally found extremely high coverage (close to 100% in some cases).
- Malaria Consortium has told us it puts more weight on post-round results, as post-round surveys are explicitly designed to achieve representative samples, whereas post-cycle surveys are designed to assess whether each program area unit met a target coverage level.
We have therefore chosen to use the post-round results to estimate the cost of delivering SMC to a child.
How Malaria Consortium’s monitoring informs our cost-effectiveness analysis
We use the data collected in Malaria Consortium’s coverage surveys in two ways in our cost-effectiveness analysis:
- As an input to calculate the cost per cycle of SMC delivered. We use estimates of the proportion of children reached in each country, alongside estimates of target populations and information on campaign costs (discussed below) to estimate the average cost to deliver one cycle of SMC to a child in Malaria Consortium-supported campaigns (approximately $1.50 as of December 2023, varying by location).115 We discuss our approach in detail in this section of our report on SMC.
- Adjustment for quality of monitoring and evaluation. Although we think that Malaria Consortium’s coverage surveys provide relatively strong evidence that a high proportion of the target population has been reached with SMC, our best guess is that the headline reported coverage figures are slightly inflated because of the concerns we identify above. To account for this, we incorporate a -2% adjustment in our cost-effectiveness analysis. This figure is based on a rough percentage best guess of the impact of the concerns we have identified rather than an explicit model.116 Because of our overall high level of confidence in Malaria Consortium’s monitoring, this adjustment is relatively low compared to some of GiveWell’s other grantees.117
2.3 Cost data for previous SMC campaigns
Malaria Consortium has shared data on costs incurred for each of the campaigns it has supported with GiveWell funding between 2018 and 2021. This includes costs incurred by Malaria Consortium itself and, in most cases, costs incurred by other actors on co-funded campaigns. See this spreadsheet, “Cost” sheets (2018-2021) for a detailed breakdown.
We use this data (alongside estimates from the coverage surveys discussed above, and estimates of the target population of 3 - 59 month olds in locations where Malaria Consortium supports campaigns) to estimate the average cost to deliver one cycle of SMC to a child in Malaria Consortium-supported campaigns. See this section of our report on SMC for a detailed discussion of our method and this spreadsheet for our calculations.
In summary:
- As of December 2023, we estimate that it costs approximately $1.50 to deliver one cycle of SMC to a child in Malaria Consortium-supported programs (varying by country, from $1.40 in Nigeria to $1.81 in Chad).118
- Our main uncertainties about the data we have seen are:
- Lack of information on other actors’ spending in Togo. We use a weighted average figure for other countries ($1.50) in place of a cost-per-cycle estimate in Togo. This is because Malaria Consortium co-funds its program in Togo with UNICEF and the Global Fund119 , but information on these other actors’ costs is not included in our cost analysis (we received information on these costs in August 2023, but have not yet incorporated it into our cost analysis). We therefore expect our estimate in Togo to be less accurate than in other locations.
- Target population data. Our estimates of the number of children reached are based on data on the number of children aged 3 - 59 months in districts where Malaria Consortium works. These figures are based on administrative data from national governments, often old census data that has been adjusted to account for population growth. These may be inaccurate for various reasons (e.g., differential population growth at the local level, migration, or internal displacement).120
2.4 Annual report on program activities
Malaria Consortium shares a narrative report each year with GiveWell with a detailed summary of its program activities in the previous year (see here for the 2022 report).121 This includes detailed information on topics including:
- Operational aspects of its program
- The number of community distributors trained and children reached in the locations where it supports SMC
- Activities supporting Malaria Consortium’s core SMC program, such as digitalization of campaign tools and operational research
We find this report valuable because it synthesizes a high volume of descriptive information on Malaria Consortium’s SMC program. This helps us understand its program in detail and helps contextualize the other information that we receive from Malaria Consortium.
3. Qualitative assessment
In theory, our recommendations are maximizing for one thing: total improvement in well-being per dollar spent. This is what our cost-effectiveness estimates intend to capture.
In practice, there are costs and benefits that we do not observe and are not estimated in our models. We make qualitative assessments to account for these unmodeled costs and benefits. We then use these assessments alongside our cost-effectiveness estimates to inform our funding recommendations.
As one tool for thinking through and communicating our impressions that aren't captured in our cost-effectiveness estimates, we assess each organization on eight dimensions on a four-point scale (“Stands out”; “Relatively strong”; “Average”, “Relatively weak”). We believe our top charities are exceptional relative to the majority of organizations and so these assessments are intended to capture differences among GiveWell’s top charities, rather than absolute rankings among all charitable organizations. Our latest assessment of Malaria Consortium (for 2023) is in the table below.
| Dimension | What does this capture? | Assessment |
|---|---|---|
| Responses to our questions | When we ask the organization a question, do its answers generally either indicate that it has thought through the question before or show us why getting an answer is not important to understanding its work? | Relatively strong |
| Prioritization discussions | Do the organization's explanations about how it allocates funding among different locations and program participants seem to be aimed at maximizing its impact per dollar? | Relatively strong |
| Self-improvement and attitude toward mistakes | Does the organization proactively share information with us and publicly about mistakes it has made? | Relatively strong |
| Role in field | Is the organization producing research aimed at informing policymakers or other implementers? Does it participate in global conversations about its field of work? | Stands out |
| Responsiveness | Does the organization send us information by mutually agreed-upon deadlines? Is it responsive to our emails? | Stands out |
| Giving us feedback | Does the organization catch our mistakes and let us know, thus improving our research? Does the organization make useful suggestions for how we could improve our research process and cost-effectiveness models? | Relatively strong |
| Quality of information shared | Have the documents that the organization has shared with us contained significant errors? Has the organization told us things that were inaccurate? Has the information provided been easy to interpret and use? Have the organization's projections of when it would achieve its goals generally been accurate? | Stands out |
| Incorporating feedback from participants and last mile providers | How does the program collect feedback from program participants and from program implementers, i.e. those directly delivering the program? How does the program incorporate feedback to improve service delivery? | Not yet assessed122 |
Overall, our assessment of Malaria Consortium is highly positive, even compared to our other top charities, and we believe it stands out on a number of factors. Some of the main factors informing our assessment are:
Role in field (stands out)
- Malaria Consortium is a leading organization in the SMC field. It was the lead implementing organization in the ACCESS-SMC project (which scaled up SMC for the first time across the Sahel),123 has played a leading role in bringing SMC to new geographies outside the Sahel since 2020-2021,124 and is a core member of the SMC Alliance (a coalition of organizations involved in SMC campaigns).125
- Malaria Consortium estimates that it supported delivery of SMC to approximately 24 million children in 2022, around half of the total population who received SMC worldwide that year.126
- Our impression is that this experience gives Malaria Consortium a high level of expertise in delivering SMC. Our best guess is that this contributes to its delivery of high-quality SMC programs.
- Malaria Consortium’s program also spans all aspects of the SMC campaigns it supports, including planning, training community distributors, SMC delivery, and monitoring. We would guess that this gives it strong oversight and a greater ability to address problems when they occur.
Quality of information shared (stands out)
- The information we have received from Malaria Consortium has generally been very detailed, and easy for GiveWell to understand and interpret (e.g., in the annual report discussed above). This gives us confidence that we understand its SMC program in detail.
- We see it as a positive that Malaria Consortium draws attention to potential weaknesses and problems in its program. For example, it has previously shared coverage survey reports highlighting limitations in its monitoring methodology.127 We find this information valuable because we aim to account for possible methodological weaknesses in our cost-effectiveness analysis.
Other factors
- Malaria Consortium is a large NGO with in-country presence and staff in many countries with the highest malaria burdens. We believe that it has a strong track record of scaling up programs quickly and reaching high coverage when it has the funding to do so. For example, Malaria Consortium expanded the number of children reached with SMC through GiveWell-directed funding from approximately 7.14 million in 2020 to approximately 16.08 million in 2022.128
- Our understanding is that Malaria Consortium sees its role in SMC programs as supporting each country’s national malaria program.129 Our best guess is that this contributes to it having strong working relationships with the national malaria programs it collaborates with, and leads to improved delivery of SMC.
- When we have requested feedback on Malaria Consortium from national malaria programs and other SMC stakeholders, we have heard positive (and often very strongly expressed) comments. This includes feedback that Malaria Consortium is a good partner to work with, that it delivers high-quality programs, and that it conducts high-quality research.
- Malaria Consortium also has dedicated research and technical staff, providing capacity to conduct large-scale studies (like the RCTs of SMC in Mozambique and Uganda we discuss above), and high-quality monitoring.
4. What do you get for your dollar?
GiveWell recommends interventions and charities that we believe are cost-effective in the sense of saving or improving lives as much as possible for as little money as possible. To estimate cost-effectiveness, we produce a cost-effectiveness analysis (“CEA”) for each of the interventions we consider.
We summarize the full reasoning behind our cost-effectiveness analysis for Malaria Consortium in our separate report on SMC. In summary, as of December 2023, we think:
- It costs approximately $2,000 to $7,000 to avert a death in areas where GiveWell currently funds Malaria Consortium to deliver SMC campaigns. In simple terms, this is because:
- There is strong evidence that SMC reduces malaria cases.
- SMC is very cheap to deliver (around $1.50 per cycle).
- Children are very unlikely to be able to access SMC other than through mass campaigns like the ones Malaria Consortium delivers.
- In addition to averting child mortality, we think SMC probably provides substantial additional benefits including increased income in later life.
See our SMC report for more details.
5. Previous Malaria Consortium grants
6. Sources
- 1
"Established in 2003, Malaria Consortium is one of the world’s leading non-profit organisations specialising in the prevention, control and treatment of malaria and other communicable diseases among vulnerable populations. Our mission is to save lives and improve health in Africa and Asia, through evidence-based programmes that combat targeted diseases and promote universal health coverage. Malaria Consortium’s Head Office is based in London, UK." Malaria Consortium, "About Us."
- 2
Malaria Consortium, comments on a draft of this page, December 2022.
“Established in 2003, Malaria Consortium is one of the world’s leading non-profit organisations specialising in the prevention, control and treatment of malaria and other communicable diseases among vulnerable populations.” Malaria Consortium, "About Us."
- 3
In addition to the grant to distribute ITNs, GiveWell also provided Malaria Consortium with two separate grants for monitoring and evaluation of those campaigns, one for each state:
- 4
“SMC is the intermittent administration of a curative dose of antimalarial medicine during the malaria season to asymptomatic children, regardless of whether the child is infected with the malaria parasite – that is, asymptomatic children are not tested for malaria before SMC administration. The objective of SMC is to establish antimalarial drug concentrations in the blood that clear existing infections and prevent new ones during the period of greatest malaria risk. SMC is recommended in areas of highly seasonal P. falciparum malaria transmission… The priority target areas for SMC implementation are those where:
- P. falciparum malaria transmission is highly seasonal and the majority (>60%) of clinical malaria cases occur within 4 consecutive months – where data on malaria from the health management information system are unreliable, rainfall data could be used as a proxy for seasonality in incidence (at least 60% of annual rainfall in 4 consecutive months); and
- the clinical attack rate of malaria (without SMC) is at least 0.1 episodes per child during the transmission season in the target group.”
- 5
Children with confirmed malaria are not given SMC. In locations where rapid diagnostic tests (RDTs) and antimalarial treatments are available, children with danger signs for malaria (including a fever) are tested, and given SMC if the test is negative. In locations where tests and antimalarials are not available, all children with malaria danger signs (including a fever) are not given SMC and are referred to a health facility for appropriate care. Malaria Consortium, comments on a draft of this page, November 9, 2023.
“SMC should not be given to:
- a child with an acute febrile illness or a severe illness – these children need to be referred to the nearest health facility for appropriate care (or tested and, if positive for malaria, treated on the spot with an antimalarial in countries where rapid diagnostic tests and ACT are available in the community as part of the SMC campaign;
- a child taking co-trimoxazole (e.g. HIV-positive child receiving co-trimoxazole prophylaxis);
- a child who has received a dose of either SP or AQ during the previous 4 weeks; or
- a child who is allergic to either SP or AQ."
- 6
“WHO recommends that medicines used as first- or second-line malaria treatment in a country not be used for chemoprevention in that country. The combination of SP+AQ is currently recommended for SMC for the following reasons.
- In the clinical trials that provided the evidence base for WHO recommendations, SP+AQ conferred greater protection than other medicine combinations.
- There are no indications that the chemoprevention efficacy of SP+AQ is diminishing in Africa.
- The SP+AQ regimen is well tolerated and relatively inexpensive.
- The SP+AQ regimen confers protection for 28 days.”
- 7
“The following considerations apply in areas where SMC is deployed.
- The 28-day interval should be respected between cycles – that is, a child who is treated on day 1 of the first SMC cycle needs to be treated on day 1 of the following cycles.
- SP and the first dose of AQ should be taken on the first day of treatment, under directly observed therapy (DOT1). The second and third doses of AQ should be given over the next 2 days by the caregiver. Caregiver adherence to the 3-day regimen can be reinforced through appropriate health communication and community engagement. The delivery of all three doses under directly observed therapy (DOT3) is an option, although cost-effectiveness data for DOT3 are lacking.”
- 8
“In a given area, SMC medicines are typically distributed over a period of four or five days. This is called the ‘distribution period’. SMC distribution is repeated in four or five monthly cycles over the course of the high transmission season. All SMC cycles in a given year are referred to as a ‘round’ of SMC.” Burkina Faso, Chad, Mozambique, Nigeria, South Sudan, Togo and Uganda. See Malaria Consortium’s seasonal malaria chemoprevention programme: Philanthropy report 2022, p. 21.
Note the 2023 WHO recommendation allows for 3 cycles of SMC, but no locations with only 3 cycles are currently funded by GiveWell. “SMC should be implemented during the peak malaria transmission period, when the incidence of malaria is highest. SMC courses should be given at 28-day intervals, beginning at the start of the transmission season and continuing for 3–5 cycles, depending on the local context.” World Health Organization, Seasonal malaria chemoprevention with sulfadoxine–pyrimethamine plus amodiaquine in children: a field guide, 2nd edition, 2023, p. 2
- 9
- 10
“Seasonal malaria chemoprevention (SMC) is recommended in areas of highly seasonal malaria transmission across the Sahel sub-region . A complete treatment course of amodiaquine plus sulfadoxine-pyrimethamine (AQ+SP) should be given to children aged between 3 and 59 months at monthly intervals, beginning at the start of the transmission season, to a maximum of four doses during the malaria transmission season (provided both drugs retain sufficient antimalarial efficacy)...
…Target areas for implementation are areas where:- Malaria transmission and the majority of clinical malaria cases occur during a short period of about four months.
- the clinical attack rate of malaria is greater than 0.1 attack per transmission season in the target age group, and
- AQ+SP remains efficacious (>90% efficacy).”
- 11
See World Health Organization, World malaria report 2023, p. 64, table 7.1.
- 12
"In 2021, the malaria programs in Burkina Faso, Nigeria, and Uganda introduced five monthly SMC cycles in areas where the transmission season is slightly longer." Malaria Consortium’s seasonal malaria chemoprevention program: Philanthropy report 2021, pg. 17.
- 13
Malaria Consortium began piloting SMC in Nampula, Mozambique, where it targeted approximately 70,000 children in two districts in 2020-2021, and Karamoja, Uganda, where it targeted approximately 90,000 children in two districts in 2020-2021. It has since supported the roll-out of SMC across Nampula and Karamoja to 1.3m and 230,000 children respectively.
“An insight brief summarising lessons learnt from the first phase of the project during the 2020/21 season, when SMC was implemented in two districts of Nampula, targeting 70,000 children, was published on Malaria Consortium’s website.[50]...With support from GiveWell,[51] SMC was further scaled up to all 23 districts in Nampula during the 2022/23 season, with a total target population of 1.30 million children.
“In 2021, the first year of SMC implementation in Uganda, philanthropic funding was used to deliver SMC to 90,000 children in two districts of Karamoja. In 2022, philanthropic funding supported SMC delivery in eight districts of Karamoja, with a total target population of 230,000 children (Figure 16).” Malaria Consortium’s seasonal malaria chemoprevention programme: Philanthropy report 2022, pg. 42, 59. - 14
“The original recommendation restricted SMC use to the Sahel subregion of Africa; SMC could not be recommended, at the time, in areas outside the Sahel with highly seasonal malaria transmission, such as in southern Africa, due to high levels of resistance to the medicines (SP and AQ) in those areas. The updated recommendation recognizes that countries in other parts of Africa with highly seasonal variation in malaria burden could also benefit from SMC, and that the availability of new medicines could make it a viable intervention in these areas.
The original recommendation stated that a maximum of 4 monthly doses of SMC should be given during the malaria transmission season. The updated guidance states that SMC should be given during peak malaria transmission season, without defining the specific number of monthly cycles… While the original recommendation restricted SMC use to children less than 6 years of age, the new recommendation recommends this intervention for children at high risk of severe malaria, which may extend to older children in some locations.” World Health Organization, Updated WHO recommendations for malaria chemoprevention among children and pregnant women, June 2022.
- 15
“Children in age groups at high risk of severe malaria are eligible. Malaria programmes should use local data to determine which age groups are at high risk of severe malaria. In most countries with intense seasonal malaria transmission, these are children below 5 years of age (1).” World Health Organization, Seasonal malaria chemoprevention with sulfadoxine–pyrimethamine plus amodiaquine in children: a field guide, 2nd edition, 2023, pg. 2.
- 16
Malaria Consortium notes that it only provides funding for SMC campaigns in locations supported by philanthropic funding, including GiveWell. In locations where other funding sources are used, the funding is provided by donors, not Malaria Consortium. Malaria Consortium, comments on a draft of this page, November 9, 2023.
- 17
Burkina Faso, Chad, Mozambique, Nigeria, South Sudan, Togo and Uganda. See Malaria Consortium’s seasonal malaria chemoprevention programme: Philanthropy report 2022, pg. 9.
- 18
See World Health Organization, World malaria report 2023, p. 64, table 7.1.
Note: Malaria Consortium’s seasonal malaria chemoprevention programme: Philanthropy report 2022 gives this figure at 48 million and refers to it both as the number of children “reached” with SMC (p. 3) and “targeted” with SMC (p. 12). We’re not sure why there’s a discrepancy with the WHO report or whether different definitions were used in each source. We haven’t investigated this in detail because our aim here is to present a rough estimate of the share of children reached with SMC who are supported by Malaria Consortium.
- 19
Our understanding is that this refers to all funding Malaria Consortium receives through GiveWell or other charitable donations. It excludes funding from multilateral organizations. “The majority of Malaria Consortium’s funding for SMC comes from philanthropic sources. This includes grants and donations to Malaria Consortium’s entities in the United Kingdom (UK) and the United States (US), primarily as a result of being awarded Top Charity status by GiveWell,[1,2] a nonprofit dedicated to finding outstanding giving opportunities and publishing the full details of its analysis to help donors decide where to give.” Malaria Consortium’s seasonal malaria chemoprevention programme: Philanthropy report 2022, pg. 8.
- 20
“Out of a total of 23.78 million children targeted with Malaria Consortium’s support in 2022, 15.10 million were reached exclusively with philanthropic funding. An additional 970,000 children were supported through co-funding arrangements with the Global Fund to Fight AIDS, Tuberculosis and Malaria (Global Fund), the United Nations Children’s Fund (UNICEF) and the Korea International Cooperation Agency (KOICA). Global Fund funding exclusively was used to support SMC delivery to the remaining 7.71 million children (Table 1).” Malaria Consortium’s seasonal malaria chemoprevention programme: Philanthropy report 2022, pg. 9.
- 21
Created using https://www.mapchart.net/.
- 22
For ACCESS-SMC delivery methods, see the annexes on pgs. 31-70 of ACCESS-SMC, The Cost of Seasonal Malaria Chemoprevention in the Sahel Region of Africa, January 2017. Sample quote: "A combination of 6,500 trained community distributors and 355 health facility staff (e.g. nurses and midwives) administered SMC by way of two distribution methods: door-to-door (two-person teams) and at fixed points located at health centers (one-person teams) which were in place to serve primarily as referral centers for sick children and provide SMC to children who were came to the facility. It was estimated that 90% of SMC was distributed by door-to-door teams and 10% was distributed at fixed points. To ensure the acceptability of SMC and high rates of coverage within communities, 3,483 trained community mobilizers sensitized communities on the benefits of SMC prior to and during each distribution cycle." ACCESS-SMC, The Cost of Seasonal Malaria Chemoprevention in the Sahel Region of Africa, January 2017, pg 31.
"[Delivery from pre-arranged locations in the community] was for ACCESS only. Now is mostly [household to household]." Malaria Consortium, comments on a draft of this page, October 2019.
- 23
"Some community distributors are community health workers — a recognized cadre of community-based primary healthcare workers who receive a small stipend from the government and who provide basic health services in their communities. In most countries, the majority of community distributors are volunteers recruited and trained specifically for the SMC campaign. Community distributors typically work in pairs." Malaria Consortium’s seasonal malaria chemoprevention program: Philanthropy report 2021, pg. 13.
- 24
Malaria Consortium emails, November 23, 2016 (unpublished).
Malaria Consortium, comments on a draft of this page, October 2017. - 25
"During SMC distribution, community distributors are assisted by field supervisors who receive more in-depth training on supervision and mentoring skills. Each team of community distributors should be observed by, and receive constructive feedback from, a supervisor at least once every cycle. Supervision is coordinated by health workers at the health facilities that serve as functional units for SMC distribution, sometimes with support from community health workers. Malaria Consortium staff and local, regional, and central health authorities also support the supervision of SMC implementers." Malaria Consortium’s seasonal malaria chemoprevention program: Philanthropy report 2021, p. 14.
- 26
“Out of all children supported with philanthropic funding in 2022, 21 percent lived in areas that received SMC for the first time that year, primarily in Nigeria and Mozambique. Just under two-thirds of the philanthropically supported target population (62 percent) lived in areas where four SMC cycles were implemented. The remaining 38 percent received five cycles.” Malaria Consortium’s seasonal malaria chemoprevention programme: Philanthropy report 2022, pg. 35.
- 27
"In 2021, the malaria programs in Burkina Faso, Nigeria, and Uganda introduced five monthly SMC cycles in areas where the transmission season is slightly longer." Malaria Consortium’s seasonal malaria chemoprevention program: Philanthropy report 2021, pg. 17.
- 28
“Burkina Faso’s National Malaria Control Programme — Programme National de Lutte contre le Paludisme (PNLP) — adopted the implementation of five SMC cycles in 19 of the country’s 70 health districts, starting in 2019 (Figure 2). Of the countries supported by Malaria Consortium, some states in Nigeria and all SMC-implementing areas in South Sudan and Uganda also implement five cycles.” Malaria Consortium’s seasonal malaria chemoprevention programme: Philanthropy report 2022, pg. 14.
- 29
“All healthy children 3–59 months are eligible for SMC.” Malaria Consortium’s seasonal malaria chemoprevention programme: Philanthropy report 2022, pg. 21.
- 30
“A full course of SPAQ is given over three consecutive days. Malaria Consortium’s seasonal malaria chemoprevention programme: Philanthropy report 2022, pg. 20.
- 31
"Typically, all eligible children in a given area will be reached over a distribution period of four or five days per cycle, which is repeated monthly over the course of the transmission season." Malaria Consortium’s seasonal malaria chemoprevention program: Philanthropy report 2021, p. 13
- 32
See Malaria Consortium, 2018 seasonal malaria chemoprevention coverage report, Burkina Faso, Chad and Nigeria, Table 1, pg. 8 for an overview of SMC procedures.
- 33
Malaria Consortium emails, November 23, 2016 (unpublished).
- 34
"Those who have a fever or are unable to take oral medication should not receive SPAQ from community distributors, but will be referred to a qualified health worker for further assessment and testing for malaria infection using a rapid diagnostic test (RDT)." Malaria Consortium’s seasonal malaria chemoprevention program: Philanthropy report 2021, p. 13
Sick children can be referred to a qualified community health worker in Uganda. Christian Rassi, Program Director - seasonal malaria chemoprevention, Malaria Consortium, comments on a draft of this page, December 2022.
- 35
- "Day 1 SP and AQ should be administered by the drug distributor as DOT. If the child vomits or spits out the drugs within 30 minutes, a second dose should be given." Malaria Consortium, 2018 seasonal malaria chemoprevention coverage report, Burkina Faso, Chad and Nigeria, Table 1, pg. 8. "DOT" stands for "Directly Observed Treatment."
- Malaria Consortium, comments on a draft of this page, October 2019.
- 36
“A full course of SPAQ is given over three consecutive days. On the day of the community distributor’s visit to a household, one tablet of SP and one tablet of AQ are dispersed in water and administered under the supervision of a community distributor. This is called directly observed treatment (DOT). The remaining two doses of AQ are given to the caregiver to administer once daily over the next two days.” Malaria Consortium’s seasonal malaria chemoprevention programme: Philanthropy report 2022, pg. 20-21.
- 37
Malaria Consortium, comments on a draft of this page, October 2019.
- 38
"The child’s SMC Record Card is very important because: a) It shows the Role Model Caregiver the name and register number of the child, b) The child’s caregiver should always take it with them if they need to go to the health facility, c) It shows how many times the child received the SMC medicines each month, d) It is made of thick paper and is in a plastic packet, e) a, b and c, f) All of the above, [Correct answer: E.]" Malaria Consortium Quiz Answer Key.
We have seen a few versions of templates for "SMC Record Cards." The latest version that we have seen (from 2016) is here: SMC Record Card Template 2016.
- 39
“Children who spit out or vomit the medicines within 30 minutes of SPAQ administration should be re-dosed once. All healthy children 3–59 months are eligible for SMC, except those who are allergic to SP, AQ, or any other sulfacontaining medicines. Children who received SP or AQ within the past month should also not receive SMC. Those who have a fever or are unable to take oral medication should not receive SPAQ from community distributors, but will be referred to a qualified health worker for further assessment and testing for malaria infection using a rapid diagnostic test (RDT). Children who test negative for malaria should receive SPAQ if deemed safe by a health worker.” Malaria Consortium’s seasonal malaria chemoprevention program: Philanthropy report 2021, p. 13.
Malaria Consortium told us that caregivers are able to visit CDs to request replacement doses if their child vomits. Malaria Consortium staff, conversations with GiveWell, November 7 and November 9, 2016.
- 40
Malaria Consortium, comments on drafts of this page, October 2019 and October 2020.
- 41
Diagram from Malaria Consortium, Coverage and quality of seasonal malaria chemoprevention supported by Malaria Consortium in 2021, p. 9.
- 42
- “SMC campaigns are implemented under the leadership of national malaria programmes and through countries’ existing health system structures. Consequently, Malaria Consortium’s role in supporting SMC varies from country to country. However, we generally provide technical and operational support on all the components that together make up SMC as a public health intervention: administration of SMC medicines; planning and enumeration; procurement and supply management; community engagement; training; case management and pharmacovigilance; supervision; and monitoring and evaluation.” Malaria Consortium’s seasonal malaria chemoprevention programme: Philanthropy report 2022, pg. 3.
- Diego Moroso, Project Director for ACCESS-SMC at Malaria Consortium, email to GiveWell, August 14, 2017 (unpublished).
- Malaria Consortium, comments on a draft of this page, October 2019.
- 43
Malaria Consortium notes that it only provides funding for SMC campaigns in locations supported by philanthropic funding, including GiveWell. In locations where other funding sources are used, the funding is provided by donors, not Malaria Consortium. Malaria Consortium, comments on a draft of this review, November 9, 2023.
- 44
"An SMC campaign typically begins around five months before the start of the annual SMC round. This involves agreeing campaign dates and modalities at the national and state levels, as well as reflecting on lessons learned in previous years to inform adaptations to the SMC intervention tools and protocols. Micro-planning at the subnational level is conducted about four months before the start of the SMC round, including budgeting based on detailed enumeration of the target population at the subnational level, required personnel, and commodities." Malaria Consortium’s seasonal malaria chemoprevention program: Philanthropy report 2021, p. 11.
- 45
This may include "training materials, monitoring & evaluation forms, bags, t-shirts etc. used by CDs, water and cups for SMC administration." Malaria Consortium, comments on a draft of this page, October 2019.
- 46
“Once the medicines have been produced, they need to be transported to Africa, preferably by sea owing to the lower freight cost, or by air at a higher cost if the consignment is more urgent. The process of procuring the medicines and coordinating the transport to central-level warehouses in the countries we support is led by Malaria Consortium’s global operations team (Spotlight 5). As a UK-based organisation, our drug and medical supply chain management is subject to Good Distribution Practice regulations and standards set out by the UK Medicines and Healthcare Products Regulatory Agency. Once the medicines have passed customs and quality assurance procedures, they are distributed further using country-level supply chain mechanisms, typically to the district level, the lowest administrative level where suitable storage facilities exist.” Malaria Consortium’s seasonal malaria chemoprevention programme: Philanthropy report 2022, pg. 23.
- 47
“The manufacturing lead time can be up to 10 months and, consequently, orders need to be placed around one year before the start of the annual SMC round. The medicines need to be transported from the manufacturers’ production plants in China and India to ports in Africa, preferably by sea owing to the lower freight cost, or by air at a higher cost if the consignment is more urgent. Once the medicines have passed country-level customs and quality assurance procedures, they are distributed further using country-level supply chain mechanisms, typically to the state or health district level, the lowest administrative level where suitable storage facilities exist. In addition to SPAQ, SMC commodities include, for example, branded T-shirts, hijabs, bags, and pens, as well as items required to minimize the risk of COVID-19 infection among SMC implementers and communities, such as face masks and hand sanitizer. Last-mile distribution—the transport of commodities to the health facilities that serve as functional units for the SMC campaign —happens just before the start of SMC distribution. This can be challenging due to poor infrastructure and limited storage facilities. Supply management also involves reverse logistics, which is the process of transporting SMC commodities back to a central warehouse at the end of the cycle or annual round.” Malaria Consortium’s seasonal malaria chemoprevention program: Philanthropy report 2021, p. 12.
- 48
"SMC implementers are trained through a cascade model beginning at the national level about two months before the start of the annual SMC round, with each cadre of trainers subsequently training the next lower level of trainers and learners. Community distributors are typically trained at the health facility level. SMC training includes modules on identifying eligible children, referring sick children to a health facility, administering SPAQ safely, recording SPAQ administration, interpersonal communication, and safeguarding. In some countries, separate trainings are conducted on supply chain management and health education." Malaria Consortium’s seasonal malaria chemoprevention program: Philanthropy report 2021, p. 12.
- 49
"During SMC distribution, community distributors are assisted by field supervisors who receive more in-depth training on supervision and mentoring skills. Each team of community distributors should be observed by, and receive constructive feedback from, a supervisor at least once every cycle. Supervision is coordinated by health workers at the health facilities that serve as functional units for SMC distribution, sometimes with support from community health workers. Malaria Consortium staff and local, regional, and central health authorities also support the supervision of SMC implementers." Malaria Consortium’s seasonal malaria chemoprevention program: Philanthropy report 2021, p. 14.
Some additional details on this activity: "Malaria Consortium developed all original training material for ACCESS-SMC [...] We could say that now the materials are pretty settled, with only minor changes / improvement happening yearly or less, based on experience from the field. We co-organize central trainings with [National Malaria Control Programs], and we then support and oversee cascade trainings (always in joint MC/NMCP teams – CRS teams in former CRS countries under ACCESS-SMC)." Diego Moroso, Regional Project Director for ACCESS-SMC at Malaria Consortium, email to GiveWell, August 14, 2017 (unpublished)."We do continue to revise tools – mainly with a view to simplifying them, adapting to changing guidelines and harmonizing with other implementers." Malaria Consortium, comments on a draft of this page, October 2019.
- 50
"Community engagement is an important component of SMC campaigns to ensure high acceptability of the intervention among communities, as well as to encourage adherence to the SPAQ administration protocol by caregivers. Activities include sensitization meetings with local leaders, radio spots, and town announcers disseminating relevant information before and during the campaign." Malaria Consortium’s seasonal malaria chemoprevention program: Philanthropy report 2021, p. 12.
- 51
See section 6 of Malaria Consortium’s seasonal malaria chemoprevention programme: Philanthropy report 2022 for recent examples of this research.
- 52
Malaria Consortium estimates that it targeted 23,780,000 children with SMC in 2022. Philanthropic funding accounted for 15.1m of these children and Global Fund funding accounted for 7.71m. The remaining ~1m children were targeted through co-funding arrangements:
- In 2021 and 2022, Malaria Consortium also received funding from the Korea International Cooperation Agency (KOICA) to cover some of the costs of SMC in one state in Nigeria and from The Bill and Melinda Gates Foundation to cover some of the costs of the research in Mozambique and Uganda.
- Malaria Consortium has co-funded SMC in some health districts in Burkina Faso and Togo with UNICEF and in some health districts in Togo and Uganda with the Global Fund.
See Malaria Consortium’s seasonal malaria chemoprevention programme: Philanthropy report 2022, pg. 10, Table 4.
Sources for co-funding arrangements:
- KOICA:
- "In 2021, KOICA covered the cost of SPAQ for two LGAs in Bauchi, as well as the majority of implementation costs. Philanthropic funding was used to cover international freight and some implementation costs." Malaria Consortium’s seasonal malaria chemoprevention program: Philanthropy report 2021, p. 28.
- “As in the previous year, an additional 280,000 children in two LGAs were supported by Malaria Consortium in Bauchi state in 2022, where the KOICA-funded SMC IMPACT project[58] covered the majority of costs. However, philanthropic funding was used to cover SPAQ for one cycle, as well as around 40 percent of the cost of allowances paid to SMC implementers during the annual round. The SMC IMPACT project budget was fixed in 2020, before the decision was made to implement five rather than four SMC cycles in Bauchi. Philanthropic co-funding ensures that the two SMC IMPACT LGAs receive the same number of SMC cycles as the remaining, fully philanthropically supported, LGAs in the state.” Malaria Consortium’s seasonal malaria chemoprevention programme: Philanthropy report 2022, pg. 47.
- Bill and Melinda Gates Foundation:Table 20: Malaria Consortium’s SMC expenditure using third-party co-funding, 2022. Malaria Consortium’s seasonal malaria chemoprevention programme: Philanthropy report 2022, pg. 84.
- Global Fund and UNICEF: Table 1: SMC target population supported by Malaria Consortium by funding source, 2022. Malaria Consortium’s seasonal malaria chemoprevention programme: Philanthropy report 2022, pg. 10.
- 53
Malaria Consortium’s seasonal malaria chemoprevention programme: Philanthropy report 2022, pg. 35, Table 3.
- 54
- Malaria Consortium’s seasonal malaria chemoprevention programme: Philanthropy report 2022, pg. 35, Table 3.
- See this spreadsheet for a more detailed breakdown of regions supported and children targeted with GiveWell-directed funds from 2017-2021.
- 55
“An insight brief summarising lessons learnt from the first phase of the project during the 2020/21 season, when SMC was implemented in two districts of Nampula, targeting 70,000 children, was published on Malaria Consortium’s website....The second phase of the project involved SMC delivery to 110,000 children during the 2021/22 season. With support from GiveWell, SMC was further scaled up to all 23 districts in Nampula during the 2022/23 season, with a total target population of 1.30 million children.” Malaria Consortium’s seasonal malaria chemoprevention programme: Philanthropy report 2022, pg. 42.
Seasons are given as ranges of years because the malaria transmission season in Nampula runs across calendar years (unlike the malaria transmission season in the Sahel): "In the north of the country, the malaria season typically lasts from November or December until February or March, so does not align with the calendar year." Malaria Consortium’s seasonal malaria chemoprevention program: Philanthropy report 2021, p. 24.
- 56
More in this grant page.
- 57
"In 2020, the National Malaria Control Division (NMCD) approached Malaria Consortium with a request to support an SMC implementation study in Karamoja (Figure 13) to investigate the feasibility, acceptability, and impact of SMC. The study employs a similar two-phase design as the study Malaria Consortium is conducting in Mozambique and is described in more detail in the research section below. The first project phase involved SMC delivery to around 90,000 children in two districts. Taking into account local malaria transmission patterns, five SMC cycles were implemented between May and September 2021. This was the first time SMC was implemented in the country." Malaria Consortium’s seasonal malaria chemoprevention program: Philanthropy report 2021, p. 37.
- 58
“The country’s Malaria Strategic Plan 2021–2025 recommends SMC as a prevention strategy in areas where the malaria burden is high and malaria transmission is seasonal. To test if SMC can be a feasible, acceptable and impactful intervention in South Sudan, Malaria Consortium and the National Malaria Control Programme conducted an implementation study in 2022, which is described in more detail in section 6. As part of the study, SMC was implemented targeting just under 20,000 children in one county of Northern Bahr el Ghazal state (Figure 12) in 2022, supported with Malaria Consortium’s philanthropic funding. The location for the study was selected based on the seasonal malaria transmission pattern in the region, high malaria incidence according to routine health system data and the state’s relative stability compared with other potentially suitable states where the security risk is greater.” Malaria Consortium’s seasonal malaria chemoprevention programme: Philanthropy report 2022, pg. 53.
- 59
"Budget: 1,694,339.00 (USD)" Malaria Consortium, "Support Scale up of Seasonal Malaria Chemoprevention (SMC)," 2015. We also searched the Gates Foundation's grant database to see whether it made any additional grants to Malaria Consortium for SMC work, but only saw this grant (grant page available at Gates Foundation, "Malaria Consortium").
"Malaria Consortium is implementing and assessing the feasibility of a community-based seasonal malaria chemoprevention (SMC) project in Katsina state, northern Nigeria, with funding from the Bill & Melinda Gates Foundation. Following new World Health Organisation policy recommendations on SMC, this project administers full antimalarial treatments during the malaria season in areas with highly seasonal malaria transmission, to prevent illness among children under five."
"The project’s objectives are:
- To design, in consultation with key local stakeholders, an appropriate community-based delivery system for SMC in Katsina state based on formative research, which will review aspects relating to feasibility, community acceptability, effectiveness and cost
- To launch and execute SMC delivery according to the selected delivery system and collect data on process indicators and costs
- To evaluate community acceptability, costs and effectiveness of the delivery system for SMC
- To inform future national and state plans for SMC continuation/ scale up by disseminating findings and sharing experiences with key stakeholders"
Malaria Consortium, Project Brief: Seasonal malaria chemoprevention, Katsina, pp. 1-2.
- 60
Malaria Consortium emails, November 23, 2016 (unpublished).
- "Length of project: 2012-2014 (33 months)," Malaria Consortium, Project Brief: Seasonal malaria chemoprevention, Katsina, p. 1.
- "[In 2013], Total number of children covered = 487,354"; "[In 2014], Total number of children covered = 1,112,330," Malaria Consortium, Cost analysis of the seasonal malaria chemoprevention project in Katsina state, Nigeria, pg. 20. However, compare to "A total of 487,353 treatment courses were delivered in two LGAs over three treatment cycles in the first round of SMC representing an average of 115% coverage over the three cycles. In 2014, a total of 1,078,440 treatments were provided across four LGAs over four cycles with an average of 115% administrative coverage." Malaria Consortium, Monitoring and evaluation summary Nigeria, pg. 2. Our understanding is that about 4 courses of SMC were aimed to be delivered per child, suggesting that about 350,000 to 400,000 children were targeted. (487,354 + 1,112,330) / 4 = 399,921, while (487,353 + 1,078,440) / 4 = 391,448.25.
- "The project also has a number of critical milestones:...85 percent of children targeted receive all courses of SMC in the second round," Malaria Consortium, Project Brief: Seasonal malaria chemoprevention, Katsina, g. 2.
- [Under "Process of implementation 2"]: "Selection and training of 2,500 community caregivers (CHWs) and supervisors to deliver the intervention and complete the necessary forms," Malaria Consortium, SMC presentation, May 6, 2014, Slide 13. Note: Malaria Consortium gave us an updated figure of 3,600 CDs, which is cited above.
- "Intervention area 2013:
- In consultation with the State MOH and SMCP, four LGAs were chosen
- Two for implementation of SMC and two for control in 2013
- Full implementation in four LGAs in 2014," Malaria Consortium, SMC presentation, May 6, 2014, Slide 9.
- 61
For more details on the ACCESS-SMC program, see this published study. ACCESS-SMC stands for Achieving Catalytic Expansion of SMC in the Sahel.
- 62
“UNITAID has awarded up to $67 million to Malaria Consortium to oversee the largest-yet global programme to increase seasonal malaria chemoprevention (SMC) across the Sahel region of Africa, where malaria remains the leading cause of severe illness and mortality in young children...This grant will help increase capacity and reduce prices for SMC products in the seven target countries and is expected to supply an estimated 30 million treatments every year to protect 7.5 million children, preventing around 50,000 deaths." Malaria Consortium, ACCESS-SMC announcement, May 8, 2014.
Clarifications around number of children targeted annually from Malaria Consortium emails, November 23, 2016 (unpublished).
- 63
"The original ACCESS-SMC grant was expected to end on August 31st, but Malaria Consortium secured a cost extension up to February 28, 2018, to complete the third season in [Burkina Faso, Nigeria and Chad], and carry out an endline molecular markers’ survey in the seven ACCESS-SMC countries, to track trends in parasite resistance to SMC drugs." Malaria Consortium, Summary Update, May 2018, pg. 1
- 64
"Malaria Consortium’s three year project, known as ACCESS SMC, will run across seven African countries: Burkina Faso, Chad, Guinea Conakry, Mali, Niger, Nigeria and The Gambia. ACCESS-SMC will be led by Malaria Consortium, with Catholic Relief Services as the primary sub-grantee. It will be supported by London School of Hygiene & Tropical Medicine, Management Sciences for Health, Medicines for Malaria Venture, Speak Up Africa and Centre de Support en Sante International." Malaria Consortium, ACCESS-SMC announcement, May 8, 2014.
- 65
Malaria Consortium staff, conversations with GiveWell, November 7 and November 9, 2016.
"The ACCESS-SMC partnership:
- Malaria Consortium is leading the ACCESS-SMC project, tracking its impact, managing the procurement of SMC drugs and supporting malaria control programmes to implement SMC in Burkina Faso, Chad and Nigeria.
- Catholic Relief Services is the lead-subrecipient and contributing to tracking the reach and impact of the project and supporting malaria control programmes to implement SMC in Guinea, Mali, Niger and The Gambia." ACCESS-SMC, Fact sheet, 2016, pg. 2.
- 66
- See Table 7, ACCESS-SMC, The Cost of Seasonal Malaria Chemoprevention in the Sahel Region of Africa, January 2017, pg. 19.
- See "Costs" sheet, GiveWell SMC cost per treatment estimate, November 2017.
- Malaria Consortium shared the following source with us to help explain its staff structure: ACCESS-SMC, organizational structure.
- 67
For example, the following studies evaluated the effectiveness or cost-effectiveness of SMC in the ACCESS-SMC project:
- ACCESS-SMC Partnership, Effectiveness of seasonal malaria chemoprevention at scale in west and central Africa: an observational study, 2020
- Cairns et al., Effectiveness of seasonal malaria chemoprevention (SMC) treatments when SMC is implemented at scale: Case–control studies in 5 countries, 2021
- Gilmartin et al., Seasonal malaria chemoprevention in the Sahel subregion of Africa: a cost-effectiveness and cost-savings analysis, 2012
- 68
See this spreadsheet, sheet "Past spending by category, Jan-Dec 2022."
This analysis is focused on the part of Malaria Consortium's SMC work that is funded entirely or in part by philanthropic funding, primarily GiveWell-directed funding. See Malaria Consortium’s seasonal malaria chemoprevention program: Philanthropy report 2022, Table 20, pg. 84, for other partners' funding contributions. Note that other partners' contributions are not included in the spreadsheet or in the table above, which summarizes philanthropic spending only.
- 69
$6,955,209 (staff) + $300,695 (travel) = $7,255,904. See this spreadsheet, sheet "Past spending by category, Jan-Dec 2022."
- 70
$1,617,562 (research) + $157,341 (communications & advocacy) = $1,774,903. Note: We include "Rapid Assessments" expenditures in research costs. See this spreadsheet, sheet "Past spending by category, Jan-Dec 2022."
- 71
See these rows of the accompanying spreadsheet.
- 72
Malaria Consortium has also shared information about the global SMC funding landscape with GiveWell. These funding landscape analyses include estimates of the population targeted for SMC in SMC-eligible countries each year, and estimates of the number of children in each location who are eligible for SMC but not targeted (e.g. because there is not enough funding). We interpret the estimates of the SMC-eligible population not targeted as rough proxies for the funding gap for SMC in each location.
We find this information valuable because it is one input into the adjustments to our cost-effectiveness analysis to account for other actors’ spending. Specifically, we use this information as one ingredient in our estimates of the impact of our funding for SMC on other actors’ funding decisions. These estimates are discussed in detail in our separate report on SMC.
We do not discuss this information here because:
- The analysis Malaria Consortium shares is only one component in our analysis of other actors’ spending. We also use sources like the amounts granted to countries by the world’s largest external funder of malaria control programs, the Global Fund, and conversations with stakeholders (e.g., national malaria programs) about their malaria control priorities, to estimate the likelihood that Malaria Consortium’s SMC funding is causing other actors to spend more or less on SMC than they otherwise would.
- We do not have permission from Malaria Consortium to publish the information it has shared.
- 73
See this section of our analysis of Malaria Consortium’s coverage surveys.
- 74
These figures summarize coverage from Malaria Consortium’s post-round surveys (after the end of the whole SMC season) rather than its post-cycle surveys (which take place after each monthly cycle), as we expect these surveys to be more accurate. See this section of the review for further details.
- 75
See this section of our analysis of Malaria Consortium’s coverage surveys.
- 76
Our estimate of the number of children reached based on post-round surveys differs from our estimate based on post-cycle surveys by 2% in 2017, 13% for 2018, 24% for 2019, 9% for 2020, and 6% for 2021. In all years except 2017, the post-cycle surveys produce a higher estimate. See this sheet of our analysis (‘Difference’ rows) for our calculations.
See this section of the review for further details on our best guess about why the survey results differ.
- 77
“Several limitations may be noted, however. First, target populations used for calculation of administrative coverage were estimated on the basis of official population figures, which were often based on outdated national census data and adjusted for projected population growth. Estimates of population sizes may not adequately reflect population movements due to migration or internal displacement." Malaria Consortium, Coverage and quality of seasonal malaria chemoprevention supported by Malaria Consortium in 2021, p. 56.
- 78
In 2017, Malaria Consortium supported one region in Guinea Bissau with SMC but did not conduct a coverage survey. "Due to the size of the country, and the expected transient nature of Malaria Consortium support there, it was decided not to include any coverage surveys in Guinea Bissau." Malaria Consortium, Summary Update, May 2018, pg. 6.
We do not view this as a major limitation, as a relatively small portion of Malaria Consortium's spending in 2017 went to this program. See this row of the accompanying spreadsheet.
- 79
Malaria Consortium conducts two types of coverage surveys: post-cycle and post-round surveys. It has conducted a post-round survey after each SMC round supported with GiveWell-directed funds, though the 2021 post-round survey in Burkina Faso was not nationally representative (see cell note in cell A43 here). Malaria Consortium also did not conduct a post-cycle survey for each of the cycles in those rounds. See here for details on which post-cycle surveys were conducted in 2021, the most recent year of surveys that we have analyzed.
Large-scale programs Malaria Consortium has supported with GiveWell-directed funds include:
- Burkina Faso, Chad, and Nigeria, since 2017. See Malaria Consortium, Financial summary, September 2017 for 2017, and see here for 2018-2021.
- Mozambique and Togo since 2020. See here. The Mozambique SMC season spans calendar years, so though spending was incurred in 2020, we did not see coverage results until 2021. "The SMC pilot in Mozambique applies the standard SMC implementation model that has been tried and tested in the Sahel. Four monthly SMC cycles consisting of three-day courses of SPAQ were delivered door-to-door to children 3–59 months by community distributors during the peak malaria season, which in northern Mozambique lasts from November to February. Unlike in west and central Africa, SMC implementation activities in Mozambique, therefore, do not align with the calendar year.” Malaria Consortium’s seasonal malaria chemoprevention program: Philanthropy report 2020, p. 27.
- Uganda since 2021. See here.
We have not yet reviewed coverage surveys from Malaria Consortium’s small pilot program in South Sudan, which began in 2022. For more details on the program, see Malaria Consortium’s seasonal malaria chemoprevention programme: Philanthropy report 2022, pgs. 53-55.
- 80
See this section of our accompanying spreadsheet.
- 81
"To-date, SMC has only been implemented in Mozambique as part of the implementation study conducted by Malaria Consortium and PNCM, supported by philanthropic funding. Based on the promising study results so far, we hope to begin the gradual scale-up of SMC in Mozambique during the 2022/23 season." Malaria Consortium’s seasonal malaria chemoprevention program: Philanthropy report 2021, p. 26.
"In 2020, the National Malaria Control Division (NMCD) approached Malaria Consortium with a request to support an SMC implementation study in Karamoja (Figure 13) to investigate the feasibility, acceptability, and impact of SMC. The study employs a similar two-phase design as the study Malaria Consortium is conducting in Mozambique[.]" Malaria Consortium’s seasonal malaria chemoprevention program: Philanthropy report 2021, p. 37. - 82
Malaria Consortium started conducting post-cycle surveys in 2017 in order to determine whether surveys conducted closer to the time of SMC delivery would yield different coverage rates than surveys conducted at the end of the round (four or more months after the first SMC cycle of the season) and to identify and respond to program delivery issues during SMC campaigns.
- "In 2017, due to concern about methodology and especially linked to potential recall bias, we reorganized the framework as a series of 4 surveys post each cycle." Diego Moroso, Regional Project Director for ACCESS-SMC at Malaria Consortium, email to GiveWell, May 8, 2018.
- "In previous years, only one coverage survey was conducted at the end of the annual SMC round to measure coverage achieved by Malaria Consortium’s SMC program. This meant that administrative data had to be relied on to gauge coverage while the campaign was ongoing. As gathering and compiling administrative data is a time-consuming process, this limited the program’s capacity to take evidence-based corrective action and make adaptations to the intervention during the campaign. For this reason, Malaria Consortium decided to implement coverage surveys following each of the four SMC cycles in each of the three implementation countries." Malaria Consortium, 2018 seasonal malaria chemoprevention coverage report, Burkina Faso, Chad and Nigeria, pg. 12.
- "End-of-cycle (EoC) surveys employing the lot quality assurance sampling methodology following cycles 1 to 3 to enable implementing teams to identify areas of low coverage and rapidly take corrective actions to improve SMC delivery in subsequent cycles." Malaria Consortium, 2019 coverage report: seasonal malaria chemoprevention in Burkina Faso, Chad and Nigeria, pg. 4.
- 83
See this spreadsheet, "Methods" sheets for Nigeria, cells G10, for auditing measures introduced in Nigeria.
For auditing measures introduced into post-round surveys in Mozambique, see here. For Uganda, see here. - 84
The audit found that only ~3-4% of interviews being done incorrectly or results being fabricated. We do not see this as a major concern. See here.
- 85
"End-of-cycle (EoC) surveys employing the lot quality assurance sampling methodology following cycles 1, 2, and 3 (where possible) to enable implementing teams to identify areas of low coverage and other issues in SMC delivery, and to rapidly take corrective actions to improve SMC delivery in subsequent cycles." Malaria Consortium, Coverage and quality of seasonal malaria chemoprevention supported by Malaria Consortium in 2021, p. 6.
"LQAS surveys are a comparatively simple and operationally feasible way of collecting data from randomly selected supervision areas and are designed to assess whether or not the minimum expected coverage of 80% was achieved. Aggregating data at the national or state level results in a reasonably precise measure of coverage." Malaria Consortium, Summary Narrative Report 2018, pg. 8.
This approach is intended to be quicker and cheaper than typical coverage surveys, as data collectors receive less training and ask fewer questions at each household. Malaria Consortium told us that it expected to use the post-cycle results to identify and respond to implementation issues. "An LQAS framework can be done quickly by personnel with basic training, using low inputs. Results from such enquiries can be generated in real time through less complex analysis. In other words, LQAS is a valid sampling approach for rapid surveys, which offers managers action oriented information on the spot...The proposed monitoring framework combines the advantages of both approaches. The plan would be to carry out a LQAS survey at the end of Cycles 1, 2 and 3...and then to deploy a full coverage survey at the end of Cycle 4. This methodology offers the advantage of 1) quick classification of defined supervision areas in real time, to address implementation issues as they arise and improve performance, and 2) the opportunity for independent surveyors to assess coverage with an acceptable margin of error." Diego Moroso, Regional Project Director for ACCESS-SMC at Malaria Consortium, email to GiveWell, May 8, 2018 (unpublished). - 86
“In the context of public health programs such as SMC, LQAS subdivides program implementation areas into smaller functional areas (e.g. wards or health facility catchment areas) referred to as ‘supervision areas’ (SAs). The LQAS method requires a relatively small sample per SA to allow for a hypothesis test of whether a predetermined standard for a particular indicator (e.g. percentage coverage) has been met in a given SA. Although this limits interpretation of findings at the SA level, the smaller sample size allows for surveys to be rapidly completed to inform actions for program improvements (i.e. between monthly SMC cycles.” Malaria Consortium, Coverage and quality of seasonal malaria chemoprevention supported by Malaria Consortium in 2021, p. 15.
- 87
See this spreadsheet
- 88
See this spreadsheet, "Methods" sheets for post-round surveys, rows 5.
- 89
See this spreadsheet, "Methods" sheets, rows 6.
- 90
For example: “In each compound, after obtaining consent from residents for participation in the survey, a roster of all children 3–119 months was made in SurveyCTO, and their first name, age, and sex recorded. One child 3–59 months was automatically selected at random from the roster by SurveyCTO. All questions relating to coverage related to that child, and all other questions to that child’s primary caregiver. An additional child 60–119 months was also randomly selected, if present, to allow for estimation of summary statistics for the proportion of overage ineligible children who received day 1 SPAQ in each country and Nigerian state.” Malaria Consortium, Quantitative report on seasonal malaria chemoprevention supported by Malaria Consortium in 2020, pg. 18.
- 91
See this spreadsheet, "Methods" sheets for post-round surveys, rows 7.
- 92
See this spreadsheet, "Methods" sheets for post-cycle surveys (cell E7 for 2020 and G7 for 2021 survey completion rates in Burkina Faso, Chad, and Nigeria; cell C7 for 2021 survey completion rate in Togo). In Plateau State in Nigeria in 2021, only 58% of targeted households were interviewed. We do not know why this rate was lower than those in other Nigerian states. See cell G7 here.
- 93
We did not receive 2021 questionnaires, but they were based on 2020 questionnaires, which we have reviewed. "Survey forms were based on those used in 2020 in all cases." Malaria Consortium, Coverage and quality of seasonal malaria chemoprevention supported by Malaria Consortium in 2021, p. 13.
Examples from 2019 and 2020: - 94
For example: "Generic questionnaires for both types of survey were initially developed in English for Nigeria and translated into French for use in Burkina Faso, Chad, and Togo. Malaria Consortium staff in each country then made minor adaptations to the questionnaires according to the specific context, for example by changing terminology used to reflect differences in local administrative units, local usage of French, or program terminology." Malaria Consortium, Quantitative report on seasonal malaria chemoprevention supported by Malaria Consortium in 2020, pg. 13.
"Teams conducted interviews in local languages, translating from the Portuguese questionnaire on the spot." Malaria Consortium, Mozambique post-round results, 2021, p. 4. - 95
For example: "Interviews were conducted in local languages using questionnaires provided by Malaria Consortium, with data collectors translating from the English, French, or Portuguese questionnaire on the spot to caregivers and assigning responses to predefined answer categories in SurveyCTO." Malaria Consortium, Coverage and quality of seasonal malaria chemoprevention supported by Malaria Consortium in 2021, p. 19.
- 96
Caregivers report on four or five cycles of SMC, with three days of administration in each cycle.
- 97
In some cases, post-round surveys have been delayed, lengthening the recall period for responses. This was the case for the 2018 and 2019 post-round surveys in Chad and the 2021 post-round survey in Burkina Faso, all of which were conducted three months after the end of the relevant SMC rounds.
- "For example, in Chad...finding an external firm to coordinate the EoR survey took much longer than expected, resulting in the survey only being conducted in January 2019, several months after the end of the 2018 SMC round. This is much later than recommended for coverage surveys and increases the risk of recall bias." Malaria Consortium, 2018 seasonal malaria chemoprevention coverage report, Burkina Faso, Chad and Nigeria, pg. 20.
- "It was also problematic in Chad, where the EoR survey was only conducted in January due to challenges in engaging a suitable supplier." Malaria Consortium, 2019 coverage report: seasonal malaria chemoprevention in Burkina Faso, Chad and Nigeria, p. 41.
- “Recall bias is likely to increase along with time between the completion of cycle four SMC distribution and the beginning of EoR surveys. It should be noted, however, that time between the end of cycle four and the EoR surveys was shorter in 2020 than in previous years. For example, in 2020, EoR surveys took place in Chad during November in 2020, while the 2019 EoR survey took place in January 2020.” Malaria Consortium, Quantitative report on seasonal malaria chemoprevention supported by Malaria Consortium in 2020, pg. 47.
See cell G8 here for details about the 2021 delay in Burkina Faso.
- 98
Post-cycle surveys were conducted within two weeks after the relevant cycle in 2020 and 2021, and within a month after the relevant cycle in 2019.
See this spreadsheet, "Methods" sheets for post-cycle surveys, row 8. - 99
Our adjustment uses two guesses: (1) true adherence (the proportion of caregivers giving out doses on days two and three) is 10% lower than reported because of social desirability bias; (2) receiving the first dose only provides approximately 50% of the protection compared to receiving all three doses. This is a very rough guess, and further research could lead us to update this estimate up or down.
We use these guesses to adjust our estimates of how much it costs to protect one child with SMC (discussed in this section). This produces an overall adherence adjustment of 93-94% (i.e., -7% to -6%, varying by location). Our full methodology is discussed in detail in our separate report on SMC.
- 100
Our understanding is that CDs are expected to record the first dose of each cycle and the date they gave the dose on a family's record card and that the family is expected to record all other doses. We have seen a few versions of templates for SMC record cards. One version that we have seen (from 2016) is here: SMC Record Card Template 2016.
For example: "Does the child have an SMC card? (Yes or No)...Ask to see blister: present? (Y or N)...Are there tablets remaining in the blister?" Malaria Consortium, 2018 end-of-round survey report, Nigeria, pgs. 54-57. - 101
"Although coverage can also be calculated on the basis of SMC child record cards, which are given to caregivers by community distributors the first time they administer SPAQ to a child each season, their retention by caregivers has been consistently low across most areas where SMC is delivered. Moreover, information recorded by caregivers on day 2 and day 3 AQ doses administered to children at home after distributor visits may be inconsistent. As in 2020, SMC child record cards were not employed to measure coverage for the purposes of this report." Malaria Consortium, Coverage and quality of seasonal malaria chemoprevention supported by Malaria Consortium in 2021, pg. 14.
- 102
See this spreadsheet, "Methods" sheets, rows 11.
- 103
We are aware of two cases in which individuals or organizations involved in the surveys were also involved in SMC delivery. In Nigeria in 2018, post-cycle surveys were conducted by data collectors recruited by state-level health authorities, who also implemented SMC, and these data collectors may have non-randomly selected villages to survey. This may have biased data collection in two ways: (1) data collectors may have been influenced to report more positive results, and (2) because data collectors were knowledgeable about how SMC was delivered, they may have chosen to survey villages that were more likely to have received SMC. This may have biased the coverage estimates produced by these post-cycle surveys upward.
In 2021, the post-round survey in Uganda was conducted by Malaria Consortium due to cost and capacity constraints with local consultants. Because the survey was implemented by the same organization that implemented the program, we believe that this could create an incentive for the survey to report positively on program outcomes. We note that we have seen no evidence of bias in survey results, and—as noted above—we do not focus on these results in this review or in our estimate of the cost of delivering SMC to a child due to our assumption that these results are not generalizable to future years.
- "In 2018, post-cycle coverage was found to be consistently above 90%, often hovering very close to 100% in all states. However, post-cycle survey results should be interpreted with caution, as several methodological issues were documented and there were concerns over researchers being recruited from among state-level health authority staff. The post-round coverage survey found generally lower coverage figures, ranging from 92% in Katsina to 95% in Jigawa, which is likely a better reflection of actual coverage." Malaria Consortium, Summary Narrative Report 2018, pgs. 7-8.
- "The main challenge related to researchers being selected by political LGA authorities. This increases safeguarding risk and may introduce bias because state authorities have a vested interest in reporting positive implementation results. Another limitation arises from researchers’ familiarity with implementation plans. If frontline implementers focus on “easy-to-reach” areas within a supervision area (the unit of randomization) and researchers, aware of implementers’ activities, simply follow in their footsteps, this may lead to an overestimation of coverage." Malaria Consortium, Summary Narrative Report 2018, pg. 9.
- "In Nigeria, EoC surveys were carried out by data collectors recruited through state- or LGA-level health authorities, which raises concerns about the impartiality of data collectors, as their employers would have a stake in implementing a successful SMC campaign. There were also concerns that the data collectors, being familiar with the SMC campaign, may have selected villages they knew had been covered by drug distributors." Malaria Consortium, 2018 seasonal malaria chemoprevention coverage report, Burkina Faso, Chad and Nigeria, pg. 20.
- "To note: there is no evidence that this actually happened, but we discussed this as a possible limitation in the report." Malaria Consortium, comments on a draft of this page, October 2019.
- "In Uganda, meanwhile, the survey was conducted in-house due to considerations of cost and capacity of local consultancies." Malaria Consortium, Coverage and quality of seasonal malaria chemoprevention supported by Malaria Consortium in 2021, p. 22.
- 104
Malaria Consortium reports that in Chad, the 2018 post-cycle surveys and the first 2019 post-cycle survey were of low quality; issues were not mentioned in 2020 or 2021.
- "Chad: The quality of outputs from some of the selected research firms was unsatisfactory and required Malaria Consortium to carry out additional, time-consuming analyses. Despite outsourcing post-cycle surveys to different firms each cycle, deliverables did not meet our expectations, leading us to conclude that local research firms do not have the required capacity and expertise to conduct complex evaluations of coverage and quality." Malaria Consortium, Summary Narrative Report 2018, pg. 9.
- "We therefore decided to rely on independent surveys conducted by third parties. However, it should be noted that this may pose different challenges. This was particularly the case in Chad, where capacity of local research firms to execute large-scale surveys is relatively low. Due to inability to engage a suitable contractor no cycle 2 EoC survey was carried out. The EoR survey was also delayed as it was necessary to contract the same research firm from Burkina Faso which also conducted that country’s EoR survey." Malaria Consortium, 2019 coverage report: seasonal malaria chemoprevention in Burkina Faso, Chad and Nigeria, pg. 41.
- "What caused you to determine that the quality of coverage data from the first post-cycle survey in Chad was poor?...Data collection for the first end-of-cycle survey was slow and a succession of draft reports were incomplete with essential information missing. When reviewing the raw dataset, it was noted that it did not correspond exactly to the survey codebook, which may have been a result of mislabelling of variables. In addition, some variables were missing altogether. The local company that conducted the survey following cycle 3 provided better quality work and the regional firm that was commissioned for the end-of-round survey was very good." Malaria Consortium, Answers to GiveWell's questions, July 2020 (unpublished).
- 105
See this spreadsheet, "Methods" sheets for Nigeria, cells G10, for auditing measures introduced in Nigeria.
For auditing measures introduced into post-round surveys in Mozambique, see here. For Uganda, see here. - 106
3% of the interviews that were subject to GPS auditing and 4% of the interviews that were subject to audio auditing were done incorrectly or fabricated. These results were then removed from the data set, although their random selection suggests that a similar proportion of the unaudited results could have issues. For more information on the audit results, see this spreadsheet, sheet "Methods: Nigeria post-round (2019-21)", cells G10 and H10.
- 107
In 2017, survey team supervisors were instructed to repeat some interviews to ensure that interviewers administered the survey questionnaires properly. "LSHTM sends monitors into the field during surveys to provide technical assistance to research teams. In addition, survey team supervisors are instructed to repeat some interviews to ensure that interviewers are administering the survey questionnaires properly." GiveWell's non-verbatim summary of a conversation with Paul Milligan and Diego Moroso, August 2, 2017, pg. 4
Approximately 90% of repeat interviews produced the same answers for each of “number of eligible children” and "number of children treated" per household.
- Number of eligible children: (68+31+5)/(68+1+6+31+1+1+5+2) = 90.4%, Milligan, SMC in Nigeria: Coverage surveys 2017, pg. 41
- Number of children treated: (40+43+16+4)/(40+3+3+43+1+2+16+1+1+1+4) = 89.6%, Milligan, SMC in Nigeria: Coverage surveys 2017, Pg. 41
- 108
See this spreadsheet, "Methods" sheets, rows 14 and 15.
- 109
See this section of our accompanying spreadsheet.
- 110
See this section of our accompanying spreadsheet. We exclude results from Mozambique and Uganda in 2021. Because Malaria Consortium was operating at a small scale and doing implementation research in these countries, we don't expect results to provide a good indication of the coverage that it might achieve in future program years. Those results can be found here, sheet "Results: post-round coverage (2019-21)," rows 39 and 40.
- "To-date, SMC has only been implemented in Mozambique as part of the implementation study conducted by Malaria Consortium and PNCM, supported by philanthropic funding. Based on the promising study results so far, we hope to begin the gradual scale-up of SMC in Mozambique during the 2022/23 season." Malaria Consortium’s seasonal malaria chemoprevention program: Philanthropy report 2021, p. 26.
- "In 2020, the National Malaria Control Division (NMCD) approached Malaria Consortium with a request to support an SMC implementation study in Karamoja (Figure 13) to investigate the feasibility, acceptability, and impact of SMC. The study employs a similar two-phase design as the study Malaria Consortium is conducting in Mozambique[.]" Malaria Consortium’s seasonal malaria chemoprevention program: Philanthropy report 2021, p. 37.
- 111
See this sheet of our analysis ("Difference" rows) for our calculations. In 2017, post-round coverage results were higher than post-cycle results, while for the following years, post-cycle results were higher.
- 112
See this sheet of our analysis ("Difference in person-months of coverage between post-cycle and post-round" rows) for our calculations.
- 113
See this sheet of our analysis (rows 145 and 146, 199 and 200, 253 and 254, and 309 and 310) for our calculations.
- 114
For Nigeria in 2018, Malaria Consortium proposes three possible explanations for the inconsistent results: "Again, the EoR survey found slightly lower coverage in Nigeria...This difference could be a result of recall bias, data collectors’ bias or it could be due to questionnaire design." Malaria Consortium, 2018 seasonal malaria chemoprevention coverage report, Burkina Faso, Chad and Nigeria, pgs. 22-23. Of these, it appears that bias during data collection may have contributed most to skewing post-cycle results upward:
- "In 2018, post-cycle coverage was found to be consistently above 90%, often hovering very close to 100% in all states. However, post-cycle survey results should be interpreted with caution, as several methodological issues were documented and there were concerns over researchers being recruited from among state-level health authority staff. The post-round coverage survey found generally lower coverage figures, ranging from 92% in Katsina to 95% in Jigawa, which is likely a better reflection of actual coverage." Malaria Consortium, Summary Narrative Report 2018, pgs. 7-8.
- "The main challenge related to researchers being selected by political LGA authorities. This increases safeguarding risk and may introduce bias because state authorities have a vested interest in reporting positive implementation results. Another limitation arises from researchers’ familiarity with implementation plans. If frontline implementers focus on “easy-to-reach” areas within a supervision area (the unit of randomization) and researchers, aware of implementers’ activities, simply follow in their footsteps, this may lead to an overestimation of coverage." Malaria Consortium, Summary Narrative Report 2018, pg. 9.
For Nigeria in 2019-2021, our best guess is that post-round results are again a better indication of actual coverage. In 2019, post-cycle results were 34% higher than post-round results and were surprisingly high (around 95% or higher coverage, see here), which makes us concerned that there may have been issues in post-cycle survey implementation in Nigeria that Malaria Consortium has not identified and that biased results upward. This difference has decreased in subsequent years (14% in 2020; 5% in four-cycle states and 8% in five-cycle states in 2021) but has remained higher than the difference in other countries. See here, rows 195, 249, and 305.
According to Malaria Consortium, these discrepancies often result from large discrepancies in specific states. In 2020, Malaria Consortium noted: “Fourth, while results of EoC and EoR surveys were relatively consistent in Burkina Faso and Chad, this was not the case for some Nigerian states (particularly in the states of Kano, Sokoto, and Yobe), where there were significant differences in estimates for some indicators (e.g. day 1 SPAQ coverage among eligible children) in 2020 and in 2019. Efforts will be made to determine whether these differences represent a trend in coverage during the SMC round, or instead are a result of differences in quality or representativeness of EoC surveys compared with EoR surveys; if the latter, appropriate actions will be taken to ensure the representativeness of EoC surveys in 2021 and beyond.” Malaria Consortium, Quantitative report on seasonal malaria chemoprevention supported by Malaria Consortium in 2020, pg. 48. In 2021, Malaria Consortium noted that Bauchi and Sokoto states in Nigeria had the widest discrepancies in post-cycle and post-round results: "... data have recently been obtained on populations by village for Kebbi, Bauchi, and Sokoto (the latter two having the widest discrepancies between EoC and EoR results)..." Malaria Consortium, Coverage and quality of seasonal malaria chemoprevention supported by Malaria Consortium in 2021, p. 57.
For Chad in 2019, our best guess is that post-round results are a better indication of actual coverage. Post-cycle surveys were conducted after the first and third SMC cycles in Chad in 2019. Malaria Consortium reports that the first post-cycle survey in Chad was of poor quality, so we have limited confidence in the reliability of those results. The third post-cycle survey found coverage of around 98%, which we find implausibly high.
An additional consideration is that Malaria Consortium told us that it places more weight on post-round results, as post-round surveys are explicitly designed to achieve representative samples, whereas post-cycle surveys are designed to assess whether each program area unit met a target coverage level (see discussion above). This understanding is from Malaria Consortium, conversation with GiveWell, August 20, 2020 (unpublished).
- 115
See this row of our cost-effectiveness analysis.
- 116
See this row of our cost-effectiveness analysis for this adjustment. Specific factors feeding into the figure that we use include:
- Some methodological features of Malaria Consortium’s surveys may lead to coverage estimates being inflated. In particular, we would expect responses based on caregiver self-reports to inflate coverage because of social desirability bias.
- The discrepancies between the results of post-cycle and post-round surveys discussed above. Although we use results from the post-round surveys (which we would expect to be more accurate overall), these discrepancies reduce our overall confidence in the accuracy of the coverage data.
- 117
As an example, we use a -17% adjustment for our cost-effectiveness analysis for Helen Keller International’s Vitamin A Supplementation program. See this section of our review of Helen Keller for further details.
- 118
See this row of our cost-effectiveness analysis. Note: we exclude results from Mozambique in 2020-2021 and Uganda in 2021. Because Malaria Consortium was operating at a small scale and doing implementation research in these countries, we don't expect results to provide a good indication of the coverage that it might achieve in future program years. We also exclude Togo from our analysis, because we are missing data on other actors’ costs for co-funded campaigns in Togo (discussed above).
- 119
“SMC in the three eligible regions is supported by Malaria Consortium’s philanthropic funding through co-funding arrangements with the Global Fund and UNICEF (Figure 14).” See also Malaria Consortium’s seasonal malaria chemoprevention program: Philanthropy report 2022, Table 14, p. 56.
- 120
“Several limitations may be noted, however. First, target populations used for calculation of administrative coverage were estimated on the basis of official population figures, which were often based on outdated national census data and adjusted for projected population growth. Estimates of population sizes may not adequately reflect population movements due to migration or internal displacement." Malaria Consortium, Coverage and quality of seasonal malaria chemoprevention supported by Malaria Consortium in 2021, p. 56.
- 121
Previous versions of the report are available on this page.
- 122
This was added to our qualitative assessment criteria in 2023. We have not yet thoroughly assessed Malaria Consortium on this measure.
- 123
“The ACCESS-SMC partnership:
- Malaria Consortium is leading the ACCESS-SMC project, tracking its impact, managing the procurement of SMC drugs and supporting malaria control programmes to implement SMC in Burkina Faso, Chad and Nigeria.
- Catholic Relief Services is the lead-subrecipient and contributing to tracking the reach and impact of the project and supporting malaria control programmes to implement SMC in Guinea, Mali, Niger and The Gambia." ACCESS-SMC, Fact sheet, 2016, p. 2.
"ACCESS-SMC is the first project to promote the scale-up of seasonal malaria chemoprevention across the Sahel."
ACCESS-SMC, Fact sheet, 2016, p. 1.
We discuss the project in further detail in the section above. - 124
Specifically, Malaria Consortium has piloted SMC delivery in Mozambique, Uganda, and South Sudan.
“Malaria Consortium is drawing on its expertise… to establish if seasonal malaria chemoprevention (SMC) – a highly effective intervention to prevent malaria in children under five, which is currently being delivered in the Sahel region of Africa – can have similar results in countries like Mozambique.”
Malaria Consortium, “Malaria Consortium to pilot seasonal malaria chemoprevention outside Sahel for first time”.
“Until 2021, SMC had only ever been implemented in the Sahel region of West Africa…Since 2021, Uganda and Mozambique have led the way in conducting SMC implementation studies in the region for the first time.” SMC Alliance, From concept to scale: Celebrating 10 years of seasonal malaria chemoprevention, 2022, p.13.
See this section of the report for further discussion. - 125
“The SMC Alliance is a group of stakeholders involved in SMC campaigns, from National Malaria Control Programmes to international technical partners, donors, research and implementing agencies. Membership is open to all interested parties.” SMC Alliance, “SMC Alliance” page, accessed August 24, 2023.
- 126
“Malaria Consortium is a leading implementer of SMC. In 2022, we supported SMC delivery to almost 24 million children in seven countries: Burkina Faso, Chad, Mozambique, Nigeria, South Sudan, Togo and Uganda. This means around half of all children reached with SMC that year were supported by Malaria Consortium.” Malaria Consortium’s seasonal malaria chemoprevention programme: Philanthropy report 2022, pg. 3.
An estimated 49 million children were targeted with SMC in 2022. See World Health Organization, World malaria report 2023, p. 64, table 7.1. Note: Malaria Consortium’s seasonal malaria chemoprevention programme: Philanthropy report 2022 gives this figure at 48 million and refers to it both as the number of children “reached” with SMC (pg. 3) and “targeted” with SMC (pg. 12). We’re not sure why there’s a discrepancy with the WHO report or whether different definitions were used in each source. We haven’t investigated this in detail since our aim here is to present a rough estimate of the share of children reached with SMC who are supported by Malaria Consortium. - 127
For an example, see Malaria Consortium, Coverage and quality of seasonal malaria chemoprevention supported by Malaria Consortium in 2021, section 4.1, pgs. 56-57.
- 128
“In 2022, philanthropic funding or co-funding enabled Malaria Consortium to deliver SMC to 16.08 million children in Burkina Faso, Chad, Mozambique, Nigeria, South Sudan, Togo and Uganda (Table 3). This compares with 12.19 million reached with philanthropic support in 2021[37] and 7.14 million in 2020.” Malaria Consortium’s seasonal malaria chemoprevention programme: Philanthropy report 2022, pg. 35.
- 129
This understanding is based on many discussions with Malaria Consortium over time.
