Insecticide Resistance and Malaria Control - June 2016 Version
We have published a more recent version of this page. See our most recent version of this page.
In a nutshell
Insecticide resistance (defined broadly as any ways in which populations of mosquitoes adapt to the presence of insecticide-treated nets (ITNs) in order to make them less effective) is a major threat to the effectiveness of ITNs. Insecticide resistance seems to be fairly common across sub-Saharan Africa, and it seems that resistance is increasing.
The implications of current levels of insecticide resistance for the effectiveness of ITNs are unclear. There are no high-quality studies that estimate the impact of insecticide resistance on ITNs’ effectiveness in the field, partly because of ethical restrictions on testing ITNs via new randomized controlled trials (RCTs). There are anecdotal reports of ITNs failing to control malaria in some locations, but these have not been rigorously confirmed. Experts in the field of malaria control seem to agree that ITNs remain highly effective in most locations, but this conclusion seems to be based on low-quality information.
Broadly, it seems that insecticide resistance is a larger concern now than it was when we last thoroughly evaluated the evidence in 2012, but it remains difficult to quantify the impact of resistance. Our very rough best guess (methodology described in more detail below) is that ITNs are roughly one-third less effective in the areas where the Against Malaria Foundation is working than they would be in the absence of insecticide resistance. ITNs remain a highly cost-effective intervention after incorporating this discount.
We expect to have better information about the likely impact of insecticide resistance on ITNs’ effectiveness within the next year when results from RCTs testing new types of ITNs may be available.
Published: June 2016
Table of Contents
- Background on insecticide resistance
- How common is insecticide resistance? Is it increasing?
-
Is resistance making ITNs less effective?
- Why is there a lack of RCTs connecting insecticide resistance and clinical malaria outcomes?
- What other literature aims to estimate the effect of insecticide resistance on malaria control?
- Have there been cases of malaria control failure due to insecticide resistance?
- What do experts say about the impact of insecticide resistance on malaria control?
- How does insecticide resistance affect the expected cost-effectiveness of donations to AMF?
- Forthcoming literature on insecticide resistance and malaria control
- Our process for finding information on insecticide resistance
- Sources
Previous versions of this page:
Background on insecticide resistance
What is resistance?
"Resistance" can be used to refer to genetic properties of mosquitoes, to refer to behavioral properties of mosquitoes, or to refer directly to failures of mosquito control.1 In the context of malaria control, we've generally seen it used to refer to the first two; as discussed below, there is relatively little evidence of control failure due to these factors, so "resistance" is usually used to discuss specific risk factors for control failure.
The four types of resistance we've seen discussed in this context are:2
- Target-site resistance (of which "knock-down resistance" is the main type we've seen discussed): mosquitoes may develop mutations that make insecticides less effective in disabling them after reaching their "target site" (the part of the mosquito that the insecticide seeks to directly affect, often within the nervous system).
- Metabolic resistance: mosquitoes possess internal systems to detoxify foreign materials; these systems may evolve to more effectively detoxify insecticides.
- Cuticular resistance: mosquitoes' exteriors may become less prone to absorbing insecticide.
- Behavioral resistance: mosquitoes may change their behavior to avoid control efforts - for example, being more active at the times of day when humans are less likely to be indoors and protected by insecticides. The World Health Organization's 2012 Global Plan for Insecticide Resistance Management in Malaria Vectors mentions that "[a]ll behavioural traits, however, may not be negative, as they could lead mosquitoes to feed on non-human animals. It is also possible to initially mistake the decline of a vector species as behavioural resistance."3
We've seen fairly little discussion of the last two types. The World Health Organization's 2012 Global Plan for Insecticide Resistance Management in Malaria Vectors
comments:
What is the relationship between “resistance” and ITN control failure?
The presence of “resistance,” as defined above, does not necessarily imply that ITNs will be ineffective. There are multiple reasons that ITNs may retain effectiveness even against mosquitoes that are "resistant" (in the sense of demonstrating low mortality rates in laboratory settings). In addition to the fact that ITNs provide a physical barrier, the insecticide may also repel mosquitoes (and cause them to seek out other targets) even when it does not kill or fully disable them.4 In addition, it's possible that mosquitoes are still killed by the insecticide (despite reduced susceptibility) when they have enough contact with it; that insecticide may inhibit them in other ways that stop them from transmitting malaria; that resistant mosquitoes are less fit overall or less prone to transmitting malaria; or that mosquitoes that are resistant to insecticides at young ages may become less resistant as they age (and that older mosquitoes are more relevant to malaria transmission).5 We have seen one study that argues for the last of these phenomena.6
The World Health Organization writes that "[i]t is broadly accepted that different resistance mechanisms have differing capacity to cause control failure, kdr [knock-down resistance] tending to be less likely than metabolic resistance (or a combination of mechanisms) to cause control failure."7
Which insecticides are used on ITNs?
Pyrethroids are the only class of insecticide recommended by the WHO for use on ITNs.8 We do not have a strong sense of when new insecticides may be developed, but some experts estimate that no replacement insecticide suitable for use on ITNs will be available for a decade.9
How common is insecticide resistance? Is it increasing?
When evaluating current levels of insecticide resistance, it is relevant to consider both the prevalence of resistance (i.e., the existence of at least some amount of resistance, as measured by a standardized test discussed in more detail below) and the strength of resistance (i.e., the intensity of insecticide resistance in a given population of mosquitoes, which can be measured in a variety of ways and may give a better indication of whether resistance is severe enough to affect malaria control). Strong resistance in a small subset of a vector population may be a greater threat to malaria control than more prevalent weak resistance (for further elaboration, see example in footnote).10
It appears that at least weak insecticide resistance is common across sub-Saharan Africa and that insecticide resistance is becoming more prevalent. We have seen very little information about the strength of resistance in sub-Saharan Africa.
In forming our views about insecticide resistance, we rely mainly on recent commentaries by Ranson and Lissenden 2016 and Hemingway et al 2016, and on the sources cited therein. We rely on these sources because they are the most comprehensive and recent literature reviews that we were able to find (more on our process below).
Details follow.
How is insecticide resistance typically measured?
The most common test used to measure insecticide resistance is a World Health Organization (WHO)-standardized diagnostic dose assay (referred to as a “WHO bioassay” for the rest of this document). WHO bioassays measure resistance by exposing wild-caught mosquitoes to twice the minimum concentration of insecticide necessary to kill 100% of susceptible mosquitoes, and define a population of mosquitoes as resistant if the observed mosquito mortality rate is <90% (details of the testing process in following footnote).11 Our understanding is that this test is often used to determine whether insecticide resistance exists in an area (i.e., the prevalence of insecticide resistance).12 It is unclear to us whether this test is useful for determining the strength of resistance; some experts argue that it is inadequate for this purpose.13
There are a variety of other possible tests for measuring the strength of insecticide resistance. One example of such a test that we have seen was from a study in Burkina Faso which exposed mosquitoes to insecticide for varying amounts of time until 50% of the population was killed.14 Professor Hilary Ranson of the Liverpool School of Tropical Medicine told us that tests measuring the strength of resistance have not been a common part of insecticide resistance monitoring in sub-Saharan Africa to date and we have not seen much information on strength of resistance in our review of the literature.15
There are many other tests of insecticide resistance, such as experimental hut trials and cone bioassays, which may also offer useful information.16 We have not yet tried to determine which tests of insecticide resistance provide the most useful information for predicting the effect of insecticide resistance on malaria control.
Where is there insecticide resistance?
We focus our discussion on sub-Saharan Africa since the Against Malaria Foundation (AMF) is most likely to distribute nets in this region.
According to WHO bioassay data collated by IR Mapper, it appears that resistance is prevalent in all areas across sub-Saharan Africa where researchers have reported measuring resistance levels,17
though there seems to be some local variation in the prevalence of resistance (i.e., some tests found mosquito populations to be susceptible in areas nearby locations where tests found resistance):
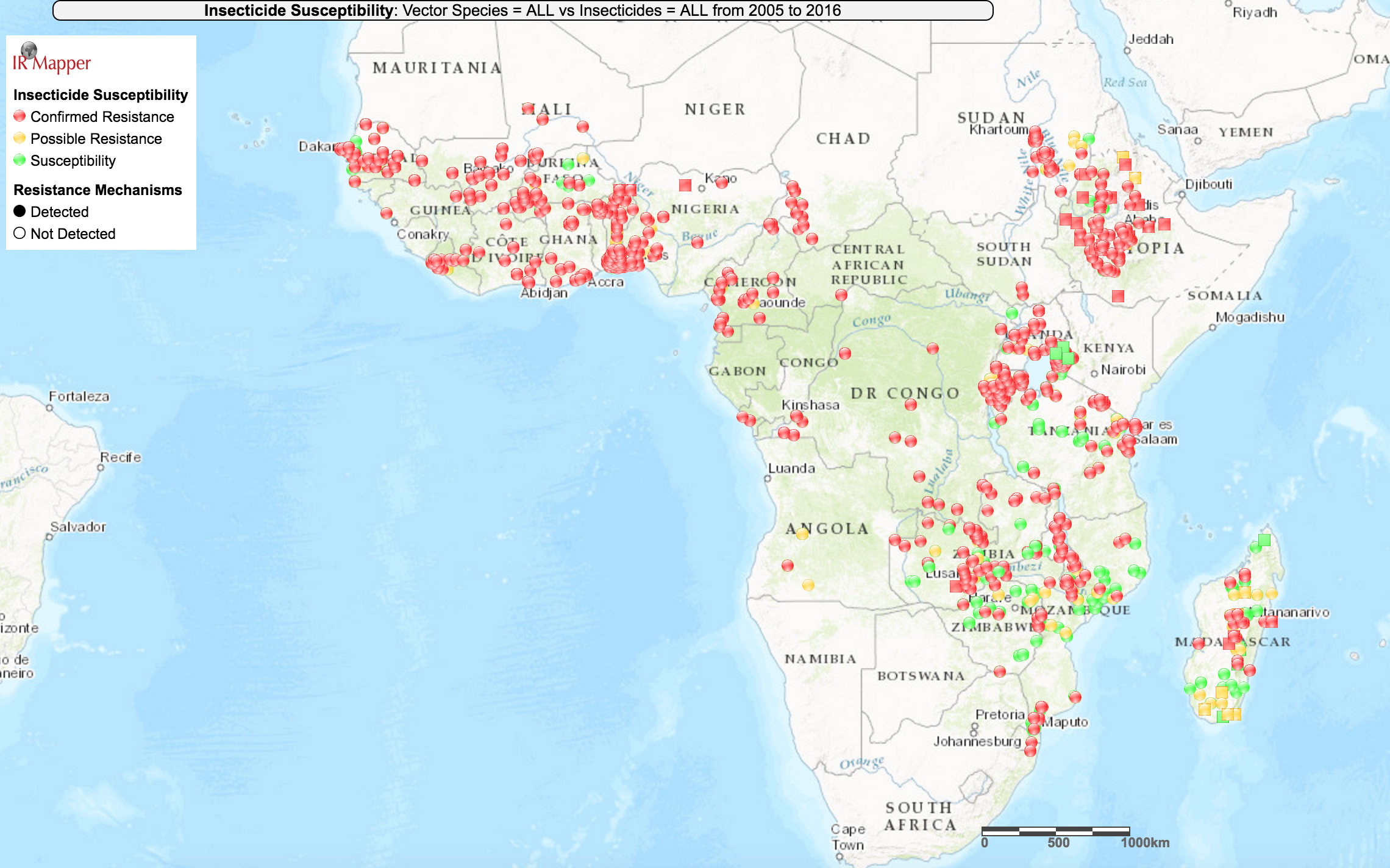
IR Mapper is a visualization tool which collates resistance data from peer-reviewed scientific papers and the reports of insecticide resistance monitoring institutions.18 We have not vetted IR Mapper's data.
Information about the strength of insecticide resistance in sub-Saharan Africa appears to be too limited to draw broad conclusions. We are aware of only three studies that explicitly attempt to measure the strength of resistance, and these studies are highly local.19 The studies aimed to measure strength of resistance either by exposing mosquitoes to increasing amounts of insecticide until 50% of the population died or by exposing them to a fixed amount of insecticide for a longer period of time until 50% of the population died; headline results from all three studies are described in the following footnote.20 In one of the studies (Toé et al 2014 in Burkina Faso) the authors found no significant difference in mosquito mortality according to WHO bioassays over a three year period, but found that strength of resistance increased 1,000-fold over the same period.21 We are unsure how meaningful these measures of strength of resistance are, and we do not know how to translate these results into likely effects on malaria control.
Because we have limited information about the best ways to predict insecticide resistance’s impact on malaria control, we do not have a strong understanding of which parts of sub-Saharan Africa are likely to be most threatened by insecticide resistance.
Is insecticide resistance increasing?
We have seen a few data points that suggest that insecticide resistance is increasing in sub-Saharan Africa. These data points, combined with the theoretical argument that insecticide resistance should be expected to increase over time as insecticides exert selective pressure on mosquitoes, lead us to believe that insecticide resistance is likely increasing. Therefore, we would guess that insecticide resistance is likely to be a greater threat to ITNs’ effectiveness in the future.
First, the following figure from Ranson and Lissenden 2016 collates WHO bioassay results for two types of pyrethroids for two species of mosquito (the dominant species that carry malaria) in sub-Saharan Africa,22
and appears to show that the average mosquito mortality rate as measured in WHO bioassays has fallen over time:
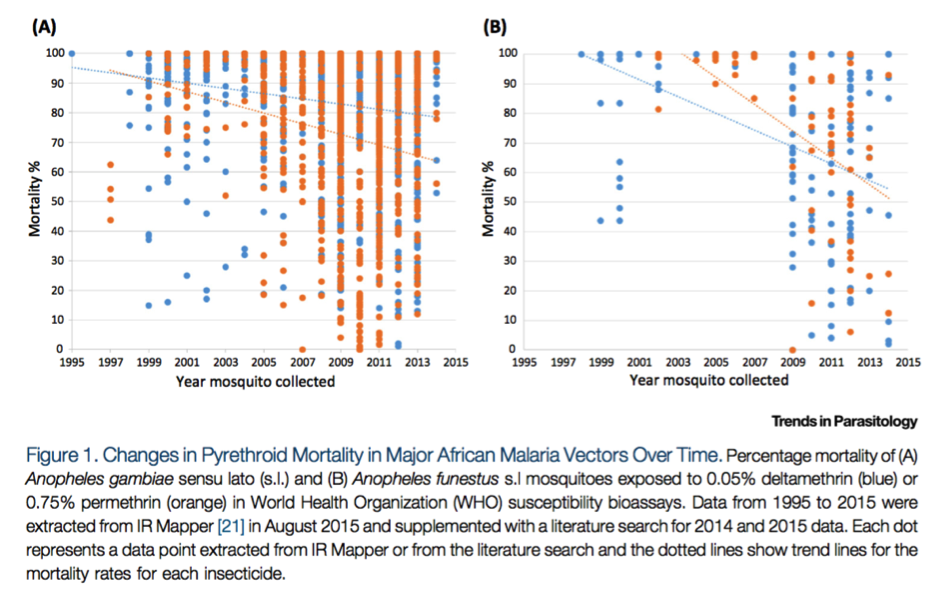
Reprinted from Trends in Parasitology, Vol 32(3), Ranson, H., and Lissenden, N., Insecticide Resistance in African Anopheles Mosquitoes: A Worsening Situation that Needs Urgent Action to Maintain Malaria Control, pp. 187-196, Copyright (2016), with permission from Elsevier.
The above figure may not be representative of the average mosquito mortality rate in sub-Saharan Africa since researchers may be more likely to measure insecticide resistance in areas where they expect it to exist, but it nonetheless seems to suggest that insecticide resistance is increasing.
Second, Ranson and Lissenden 2016 also provides a figure showing that mosquito mortality rates appear to be falling over time in a few locations (though we do not know how representative these locations are):23
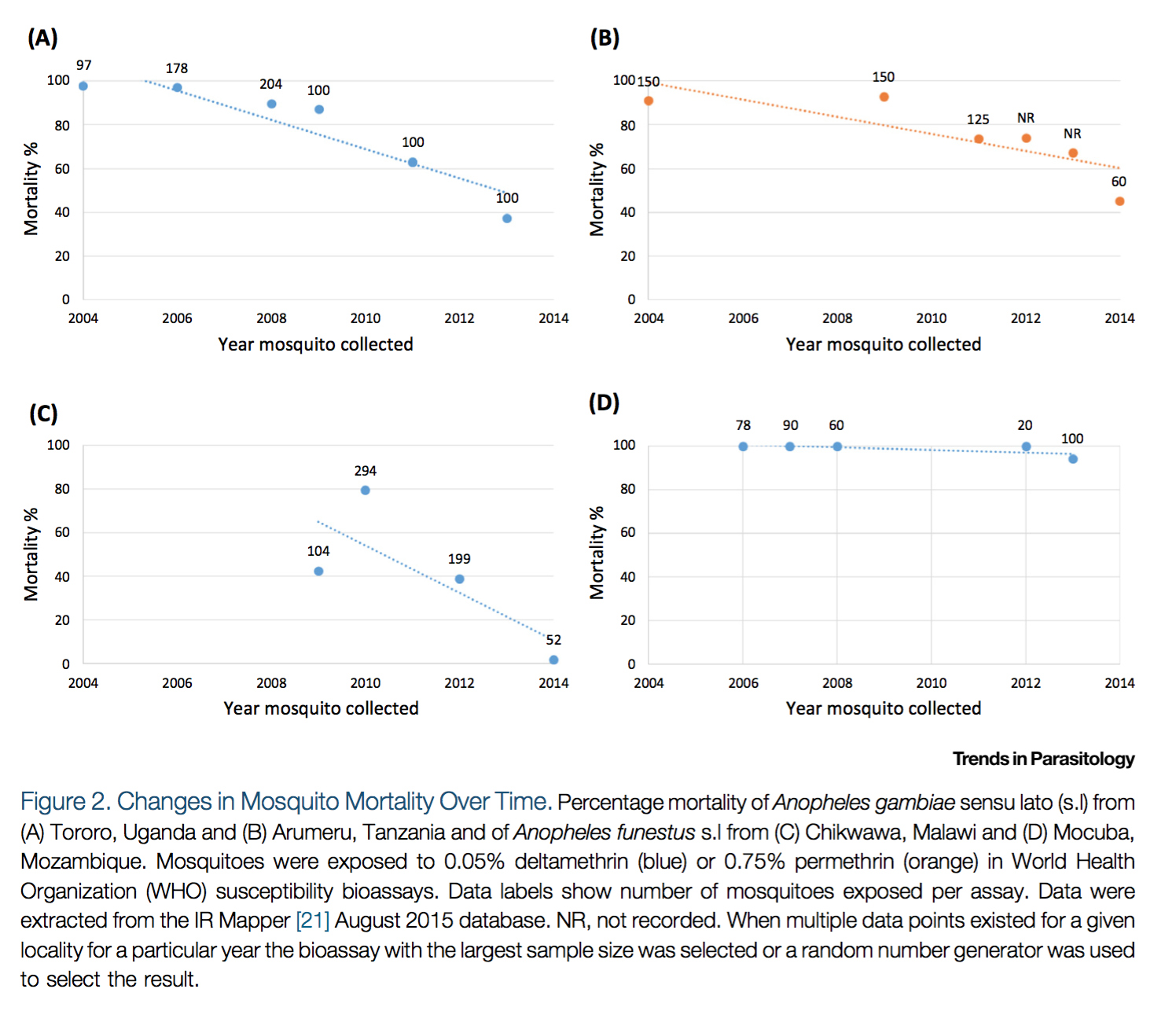
Reprinted from Trends in Parasitology, Vol 32(3), Ranson, H., and Lissenden, N., Insecticide Resistance in African Anopheles Mosquitoes: A Worsening Situation that Needs Urgent Action to Maintain Malaria Control, pp. 187-196, Copyright (2016), with permission from Elsevier.
The data presented above is from IR Mapper.24 We have not vetted IR Mapper's data.
Third, some academic commentaries that we have seen argue that resistance is increasing over time, and we have not seen any papers that argue that resistance is not increasing.25
We have limited information about whether the strength of resistance is increasing over time in sub-Saharan Africa. The limited evidence that we have seen on the strength of resistance is discussed above.
Note that this discussion covers only target-site and metabolic resistance, which make insecticides less effective in killing mosquitoes; by the nature of WHO bioassays, they do not address behavioral resistance. As discussed above, there is currently relatively little evidence regarding behavioral resistance and no clear consensus about its importance.
Is resistance making ITNs less effective?
The implications of current levels of insecticide resistance for the effectiveness of ITNs are unclear. There are no high-quality studies that estimate the impact of insecticide resistance on ITNs’ effectiveness, partly because of ethical restrictions on testing ITNs via new randomized controlled trials (RCTs). Experts generally seem to argue that ITNs remain highly effective in most locations, but this conclusion seems to be based on low-quality information.
Why is there a lack of RCTs connecting insecticide resistance and clinical malaria outcomes?
Ethical restrictions prevent running RCTs comparing ITNs to a control group without bed nets or to a control group with untreated nets (UTNs, i.e. nets without insecticide) in areas with insecticide resistance.26 Comparing ITNs to no net controls or UTNs in resistant areas is considered unethical because ITNs are a proven intervention for decreasing malaria risk and participants cannot ethically be randomized to a known lower standard of care.27 (Note we discuss below a forthcoming study which nevertheless seems to have gained ethical approval to compare ITNs and no net controls.)
What other literature aims to estimate the effect of insecticide resistance on malaria control?
We are aware of several studies that attempt to estimate the effect of insecticide resistance on malaria control. However, they all use non-randomized, low-quality study methods that we do not feel comfortable relying on for reaching conclusions about the effectiveness of ITNs.
The studies that we have seen are:
- Cohort studies, such as Lindblade et al 2015 and Asidi et al 2012, attempt to estimate the protective effect of ITN use by comparing ITN users with non-users, or ITNs in resistant versus susceptible areas.28 Lindblade et al 2015 compares malaria incidence among people who used bed nets against incidence among non-users, and attempts to adjust for potential confounding factors.29 It finds that ITNs reduced the incidence of malaria infection by about 30% compared to no bed nets in an area with moderate resistance.30 The sample size of non-users was very small and it seems challenging to adequately adjust for all confounders, so we do not feel comfortable relying on this study.31 Asidi et al 2012 compared mosquito biting and mosquito survival rates before and after treating ITNs with pyrethroids in areas with insecticide resistance and areas without resistance.32 It found that ITNs were no better than untreated nets in areas with insecticide resistance.33 This was a non-randomized study with a very small sample size (16 households), so we do not put much weight on its results.34
- Strode et al 2014 does not attempt to directly measure the effect of insecticide resistance on malaria incidence. It is a meta-analysis of cone tests, tunnel tests, and experimental hut trials (methodologies of these tests are described in the following footnote) which compares mosquito mortality and blood feeding following exposure to ITNs against mortality and feeding following exposure to UTNs at different levels of insecticide resistance.35 It finds that ITNs are more protective than UTNs at any level of resistance.36 However, the authors suggest interpreting these results with caution because of "substantive heterogeneity" in the pooled studies.37 Further, low data quality, inconsistent experimental design, and variance in methods of resistance measurement made it impossible for the authors to assess resistance's effect on malaria transmission or clinical outcomes.38 Accordingly, we did not closely investigate Strode et al 2014.
- Modeling studies that draw on correlations between insecticide resistance and malaria incidence to predict the impact of resistance. Though these studies are non-randomized and generally low-quality, they seem to be the simplest path available for very roughly estimating the impact of resistance on malaria control. We discuss some of these studies in more detail below.
Have there been cases of malaria control failure due to insecticide resistance?
There are reports of ITNs failing to control malaria in some locations due to insecticide resistance, but these have not been rigorously confirmed via high-quality studies. We have not closely examined the studies mentioned below.
Pre-2010, two longitudinal studies in Senegal (relating to ITNs and malaria treatment) and South Africa (relating to indoor residual spraying) observed an initial drop in malaria cases when malaria control interventions were implemented, followed by a rebound in malaria cases as resistance emerged.39
Recent observational evidence on ITN effectiveness in areas with resistant malaria vectors is mixed. We have seen some post-2010 observational evidence suggestive of control failure: namely, evidence that malaria case rates have stayed stable or even increased following ITN distributions in areas of Burkina Faso, Uganda and Zambia.40 Professor Hilary Ranson of the Liverpool School of Tropical Medicine told us that ITNs continue to be effective in most areas, but that there are credible reports of potential control failure in some areas.41 We have also seen discrete evidence of resistance causing possible ITN failure: a study observing resistant mosquitoes resting inside holed ITNs,42 and a study documenting early evening feeding by resistant vectors in areas with moderate coverage of malaria control interventions.43 Yet other observational evidence suggests ITNs are in fact more protective in high resistance areas.44
What do experts say about the impact of insecticide resistance on malaria control?
Our general impression is that experts in the malaria community believe that ITNs remain effective in most areas. The non-randomized studies discussed above generally seem to find that ITNs are still effective. Professor Hilary Ranson told us that ITNs continue to be effective in most areas but that there are credible reports of potential control failure.45 We have seen some experts argue that it would probably be difficult to directly associate ITN failure with insecticide resistance because of confounding factors.46
How does insecticide resistance affect the expected cost-effectiveness of donations to AMF?
Though we have not found any high-quality studies that estimate the magnitude of the impact of insecticide resistance on ITNs’ effectiveness, we believe that it is necessary to include some adjustment for insecticide resistance when projecting the cost-effectiveness of AMF.
Since we expect to have higher-quality information about the impact of insecticide resistance in the next several months (more), we have merely taken the simplest path to a reasonable-seeming insecticide resistance adjustment to use in the near term.
Our very rough best guess is that ITNs are about one-third less effective in the areas where AMF is working than they would be in the absence of insecticide resistance. In brief, the reasoning underlying this adjustment is:
- Lacking any high-quality studies connecting insecticide resistance with clinical outcomes, we must rely on the best low-quality studies on this topic. Of the studies that we’ve reviewed, modeling studies, which (in simplified terms) use correlations between levels of insecticide resistance and malaria incidence to try to predict the impact of resistance, seem least methodologically problematic though still imperfect.47
- We have seen two modeling studies which model clinical malaria outcomes in areas with ITN coverage for different levels of resistance based on experimental hut trial data.48 Of these two studies, the most recent study we have seen is unpublished (it was shared with us privately), but we prefer it because the insecticide resistance data it draws from is more recent and more comprehensive.49
- Assuming that AMF’s future distributions will occur in areas with moderate insecticide resistance on average (i.e., mosquito mortality rate of about 55%),50 the recent, unpublished modeling study would predict a reduction in the effectiveness of bed nets of about 33%.51 We have not vetted the model underlying this estimate.
- After applying various sanity checks, an estimated one-third reduction in the effectiveness of bed nets due to insecticide resistance seems in the range of a reasonable estimate.52
Our full cost-effectiveness analysis for AMF is available here.
Forthcoming literature on insecticide resistance and malaria control
The following studies measuring clinical malaria outcomes in areas with confirmed resistance and ITN distributions are currently being conducted:
- Two randomized controlled trials of new types of ITNs, which have been shown in experimental hut trials to induce higher mortality and to reduce blood feeding in resistant mosquitoes when compared to classic ITNs:53
- A cluster-RCT in rural Burkina Faso assessing whether Olyset Duo, a PPF-ITN which contains 2% permethrin (a pyrethroid) and 1% pyriproxyfen (an insect juvenile hormone mimic which reduces mosquito fecundity), provides better protection against clinical malaria in children than the pyrethroid-only Olyset ITN, which contains just 2% permethrin.54 The protocol provides that there will be ~40 clusters (20 in each arm) and at least 45 children being actively monitored for malaria in each cluster.55 Based on discussions with experts, we expect to have access to initial results from this study between June and August 2016.
- A cluster-RCT in Tanzania in 48 villages where resistant Anopheles gambiae is the dominant malaria vector. It compares (among other things) the efficacy of Olyset Plus ITNs, which contain 2% permethrin combined with 1% of the synergist PBO (piperonyl butoxide), with conventional Olyset ITNs, containing just 2% permethrin.56 The study has an estimated enrollment of 3,840 participants, began in September 2014, and has an estimated completion date of January 2017.57
- A cluster-RCT comparing (among other things) the efficacy of conventional ITNs to no net controls in south-central Ethiopia. According to the published protocol, this study will measure malaria cases based on diagnoses at health posts and will conduct contemporaneous entomological studies. It will have ~44 clusters of 35 households, and data collection will be complete in December 2016.58
- Kleinschmidt et al 2015, a four-year study in Sudan, Kenya, India, Cameroon and Benin designed to quantify the potential loss of epidemiological effectiveness of ITNs due to decreased susceptibility of malaria vectors to insecticides.59 The study includes ~300 clusters, where insecticide resistance levels are measured annually using WHO standardized methods and malaria case rates are actively detected in cohorts of children.60 With the exception of India, data collection was finalized in 2015. Data collection in India will be finalized this year.61 From correspondence with experts, we understand that results will be available in September 2016.
We expect all the RCT results to estimate the decrease in efficacy of ITNs in resistant areas. The RCT comparing ITNs to no net controls will directly measure the protective effect of ITNs in certain resistance contexts. The RCTs comparing conventional ITNs to combination ITNs where the relevant vector is 100% susceptible to the combination insecticide will estimate the loss in protective efficacy attributable to insecticide resistance. For PPF nets, we expect few confounding factors because the PPF nets being trialled in Burkina Faso are exactly the same as the conventional nets they are being compared to, except for the addition of pyriproxyfen.62 However, the PBO nets being trialled in Tanzania are made with a smaller mesh size than their conventional comparator nets, and we will need to form a view on how this affects the results when the study's analysis is available.63
Our process for finding information on insecticide resistance
- We searched Google Scholar and PubMed for papers looking at trends in insecticide resistance and, in particular, papers connecting insecticide resistance with potential malaria control failure. We relied on Ranson and Lissenden 2016 and Hemingway et al 2016 as reviews of relevant literature.
- We searched the Malaria Journal for "resistant" and "resistance," scanned for publications relevant to insecticide resistance as it relates to LLINs, and read the abstracts of those papers.
- We spoke with Prof. Hilary Ranson, a member of the Against Malaria Foundation's Malaria Advisory Group (conversation notes are available here). We also spoke with Prof. Immo Kleinschmidt, principal investigator of a multi-country study of ITN resistance’s effect on clinical malaria outcomes.
Our previous page on insecticide resistance, written in 2012, is available here.
Sources
- 1
"Molecular genotyping of resistance is the identification of the underlying genes that confer the inherited trait of resistance (15). Identification of a resistance gene provides evidence of the underlying evolutionary process. Depending on the type of resistance mechanism, this provides understanding of both the degree of resistance expressed in individual insects with the resistance gene, and the frequency of such insects in the population.
"Phenotypic resistance is the basic expression of the genetic cause of resistance, shown by a vector’s ability to resist and survive the effects of the insecticide. Phenotypic resistance is measured in a susceptibility test of vector mortality when subjected to a standard dose of the insecticide. WHO has defined phenotypic resistance as “development of an ability, in a strain of insects, to tolerate doses of toxicants, which would prove lethal to the majority of individuals in a normal population of the same species” (16). Phenotypic resistance is the phenomenon most commonly referred to in public health.
"Resistance leading to control failure - while phenotypic resistance provides an indication of the effects of resistance on the vector, the most informative way of looking at resistance is as an epidemiological phenomenon, in which resistance is identified as the cause of increasing malaria transmission. In the notion of resistance leading to control failure, evidence of resistant vectors is linked directly to the failure of vector control programmes in the field. Resistance leading to control failure can be defined as the 'selection of heritable characteristics in insect population that results in repeated failure of an insecticide product to provide intended level of control when used as recommended.' Resistance leading to control failure is the phenomenon most commonly referred to in agriculture. National malaria control programmes should not, however, wait for control failure to occur before implementing strategies to manage insecticide resistance. There is no acceptable level of control failure in public health, and waiting could result in delaying action until it is too late." World Health Organization's 2012 Global Plan for Insecticide Resistance Management in Malaria Vectors, Pg 27.
- 2
"Target-site resistance occurs when the site of action of an insecticide (typically within the nervous system) is modified in resistant strains, such that the insecticide no longer binds effectively and the insect is therefore unaffected, or less affected, by the insecticide. Resistance mutations, known as knock-down resistance (kdr) mutations, can affect acetylcholinesterase, which is the molecular target of organophosphates and carbamates, or voltage-gated sodium channels (for pyrethroids and DDT) (15, 17).
"Metabolic resistance is related to the enzyme systems that all insects possess to detoxify foreign materials. It occurs when increased or modified activities of an enzyme system prevent the insecticide from reaching its intended site of action. The three main enzyme systems are: esterases, mono-oxygenases and glutathione S-transferases. While metabolic resistance is important for all four insecticide classes, different enzymes affect different classes (15, 17).
"Although most resistance mechanisms (especially kdr resistance) have been studied for decades in previous cases of resistance, the detailed study of mono-oxygenase metabolic resistance is relatively new, and our understanding of it is fairly limited. Indeed, cases of mono-oxygenase resistance in mosquitoes were unknown before its identification in South Africa in 2000–2001 (see section 1.2.3 for details).
"As described below, metabolic and target site resistance can both occur in the same vector population and sometimes within the same individual mosquito. The two types of resistance appear to have different capacities to reduce the effectiveness of insecticide-based vector control interventions, with metabolic resistance being the stronger and more worrying mechanism (see section 1.2.3 for details).
"Behavioural resistance is any modification in insect behaviour that helps it to avoid the lethal effects of insecticides. Several publications have suggested the existence of behavioural resistance and described changes in vectors’ feeding or resting behaviour to minimize contact with insecticides. Studies in New Guinea and the Solomon Islands showed that Anopheles farauti vectors stopped biting later in the night (23:00–03:00) after the introduction of indoor DDT spraying and instead bit only in the earlier part of the evening, before humans were protected by sleeping in a sprayed room (18). In most cases, however, there are insufficient data to assess whether behavioural avoidance traits are genetic or adaptive; genetic traits could have major implications for the types of vector control interventions needed. All behavioural traits, however, may not be negative, as they could lead mosquitoes to feed on non-human animals. It is also possible to initially mistake the decline of a vector species as behavioural resistance.
"Cuticular resistance is reduced uptake of insecticide due to modifications in the insect cuticle that prevent or slow the absorption or penetration of insecticides. Examples of reduced penetration mechanisms are extremely limited and only one study has suggested correlation between cuticle thickness and pyrethroid resistance in An. funestus (19). Microarray experiments have identified two genes that encode cuticular proteins that are up-regulated in pyrethroid-resistant strains of Anopheles mosquitoes. Experience with other insects suggests that if cuticular resistance emerges in mosquitoes it could have a significant impact when combined with other resistance mechanisms." World Health Organization's 2012 Global Plan for Insecticide Resistance Management in Malaria Vectors, Pg 27-28.
- 3
World Health Organization's 2012 Global Plan for Insecticide Resistance Management in Malaria Vectors, Pg. 28.
- 4
"Although there are limited options for [resistance management] with LLINs, they may retain an effect despite increased resistance to pyrethroids. Firstly, nets provide a physical barrier against biting by mosquitoes as long as they are intact (2). Secondly, in most vector species, resistance to pyrethroids does not completely reduce the effect of the insecticide. It has also been observed that the irritancy of pyrethroids (‘hyperexcitatory response’) may reduce mosquito blood-feeding or encourage diversion to other hosts by certain vector species that do not feed exclusively on human hosts. This effect can vary, however, by species and geographical location." World Health Organization's 2012 Global Plan for Insecticide Resistance Management in Malaria Vectors, Pg 45.
- 5
- "Possible reasons for widespread insecticide resistance with no obvious impact on the effectiveness of vector control:…
- "Vectors are still killed because of multiple exposures to an insecticide in the field. Vectors may still be killed by an insecticide if they come into contact with it on many occasions within a short time (6). While a single standard dose of insecticide might not have the desired effect, multiple exposures in the field (for example landing on a sprayed surface three to four times) can lead to a higher accumulated dose of the insecticide, which may be sufficient to kill the vector. Similarly, vectors that are resistant may have a lower excito-repellent response when exposed to pyrethroids. Therefore, they may remain exposed to the insecticide for longer and receive a larger dose, and thus be killed anyway (3).
- "Older vectors are more susceptible. Metabolic resistance is in some cases expressed more strongly in young mosquitoes, but, when they are older, susceptibility returns. Therefore, insecticides still kill older mosquitoes (7). Since it is these old mosquitoes that transmit malaria, the resistance may not cause an increase in transmission.
- "Resistant vectors are less capable of transmitting malaria. It has been suggested that the ability of resistant vectors to transmit malaria might be reduced as, even if they are not killed by an insecticide within 24 hours, the insecticide might still inhibit their ability to live for the 12 days necessary to develop the malaria parasite. Alternatively, a vector's resistance to an insecticide might be offset by a lower level of infection, which is not as great a threat to the human population." World Health Organization's 2012 Global Plan for Insecticide Resistance Management in Malaria Vectors, Pg 111.
- We are aware of some entomological evidence from Kristan et al 2016 that suggests that resistant mosquitoes may be less fit to transmit the malaria parasite (see below). However, Ranson and Lissenden 2016, Pg. 8 states that "[r]elatively little is known about the fitness costs of resistance." We have not looked further into the entomological evidence about whether resistance inhibits malaria transmission.
- "We collected An. gambiae s.s. homozygous for kdr-L1014S mutation, fed them on blood samples from 42 P. falciparum-infected local patients, then exposed them either to nets treated with sub-lethal doses of deltamethrin or to untreated nets. ... Both prevalence and intensity of infection were significantly reduced in deltamethrin-exposed mosquitoes, compared to those exposed to untreated nets. With low doses (2.5–5.0 mg/m2), prevalence was reduced by 59 % (95 % CI = 22 %-78 %) and intensity by 41 % (95 % CI = 25 %-54 %). With high doses (10–16.7 mg/m2), prevalence was reduced by 80 % (95 % CI=67 %-88 %) and intensity by 34 % (95 % CI=20 %-46 %). ... Conclusions: We showed that, with locally-sampled parasites and mosquitoes, doses of pyrethroids that are sub-lethal for resistant mosquitoes can interfere with parasite development inside mosquitoes. This mechanism could enable pyrethroid-treated nets to prevent malaria transmission despite increasing vector resistance." Kristan et al 2016, Pg 1.
- "Possible reasons for widespread insecticide resistance with no obvious impact on the effectiveness of vector control:…
- 6
"The development of malaria parasites into the infectious sporozoite stage takes 10 or more days following a blood-meal [35]. The older cohort of An. gambiae s.s. used in this study (17-19 days old) could potentially harbour infectious parasites but would be more susceptible to insecticide-based control. Targeting older, and arguably more epidemiologically significant mosquitoes, has been proposed as an alternative strategy for wider malaria control in which the selection on resistance to the control agent is reduced [36]." Jones et al 2012, Pg. 8-10.
- 7
- 8
“Pyrethroids are the only class of insecticide recommended by WHO for use on longlasting insecticide-treated bednets.” Hemingway et al 2016, Pg. 1.
- 9
- “The cornerstone to IVCC’s mission is the development of three new active ingredients, each with a novel mode of action, in effect, a different way of killing mosquitoes. These will form the basis of a new generation of insecticides suitable for public health purposes.” International Vector Control Coalition - Active Ingredient Portfolio - 2016.
- “With no new insecticide class to replace the pyrethroids expected for a decade, the threat of resistance derailing malaria control has become an issue of urgency that can no longer be ignored without risking a global public health catastrophe.” Hemingway et al 2016, Pg. 1.
- 10
"Furthermore, by using prevalence of resistance as the metric, it is not possible to identify regions where resistance is likely to be posing the greatest threat to malaria control. Mosquitoes collected from site A may yield 50 % mortality in a discriminating dose assay whereas mosquitoes from site B may have been just under the threshold with 85 % mortality. This does not however mean that resistance is less of a threat in site B. The 15 % that did survive may have an extremely high level of resistance enabling them to readily survive long periods of time on a treated surface and thus potentially transmit malaria despite high coverage with vector control. In contrast, if the 50 % that survived the discriminating dose in site A have a relatively weak phenotype they will be killed when exposed to field concentrations of insecticide and thus pose less of an immediate threat." Bagi et al 2015, Pg. 2.
- 11
WHO bioassays place age-standardized mosquitoes in tubes and expose them to paper impregnated with twice the minimum concentration of insecticide known to kill 100% of susceptible mosquitoes. Exposure lasts 60 minutes, and the number of mosquito fatalities is recorded after 24 hours. The population is classified as susceptible if >98% of mosquitoes are killed, and resistant if <90% of mosquitoes are killed. Values between 90% and 98% are considered “suspected resistance,” and further tests are recommended. We do not know how the amount of insecticide that a mosquito is exposed to in a bioassay compares to the amount of insecticide that a mosquito would receive from its typical interaction with an ITN. Sources:
- “The WHO insecticide susceptibility bioassay that is the subject of these revised guidelines is a simple direct response-to-exposure test. Mosquitoes are exposed to known concentrations of an insecticide for a fixed period of time at the end of which the number of fatalities is recorded. In its present form, the test is designed to distinguish between baseline susceptibility and resistance to insecticides in adult mosquitoes. As such, the test is intended to be used as a field and laboratory surveillance tool with the limitation that it gives little information on the underlying mode(s) or mechanism(s) conferring resistance where detected.” Test procedures for insecticide resistance monitoring in malaria vector mosquitoes - World Health Organization - 2013, Pg. 4.
- "In order to be certain that all susceptible mosquitoes are killed, WHO has traditionally defined its discriminating concentrations in one of two ways, that is, as either:
- twice the lowest concentration that gave systematically 100% mortality after 60 minutes exposure and a holding period of 24 hours on a susceptible strain or a susceptible population; or
- twice the LC99.9 value as determined by baseline susceptibility testing of a susceptible strain or a susceptible population.
Table 3.1 lists the WHO recommended discriminating concentrations for insecticides used in malaria control and/or for research purposes (e.g. dieldrin) for adult malaria vectors." Test procedures for insecticide resistance monitoring in malaria vector mosquitoes - World Health Organization - 2013, Pg. 6.
- “A mortality in the range 98–100% indicates susceptibility”; “A mortality of less than 98% is suggestive of the existence of resistance and further investigation is needed”; “If mortality is less than 90%, confirmation of the existence of resistant genes in the test population with additional bioassays may not be necessary, as long as a minimum of 100 mosquitoes of EACH species was tested. However, further investigation of the mechanisms and distribution of resistance should be undertaken.” Test procedures for insecticide resistance monitoring in malaria vector mosquitoes - World Health Organization - 2013, Pg. 18.
Note that information about the three main kinds of tests for insecticide resistance – cone or tube tests (such as these), tunnel tests and experimental hut trials is available at: "Box 1. Types of Studies Included," Strode et al 2014, Pg 2.
- 12
See the data collected in IR Mapper, much of which relies on WHO bioassays.
- 13
- "World Health Organization (WHO)-standard bioassays ... don’t measure the degree of resistance. ... Recently researchers have pushed for bioassay data that is useful in predicting the impact of resistance rather than just monitoring its spread. Some (including those working on the President’s Malaria Initiative (PMI) five-country investigation of the impact of insecticide resistance on malaria vector control) have begun to use more quantitative bioassays that expose mosquitoes to increasing doses of insecticide to determine how much is needed to exterminate a given population." GiveWell's non-verbatim summary of a conversation with Hilary Ranson, Professor, Medical Entomology, Liverpool School of Tropical Medicine, March 18, 2016.
- "[WHO] standardized methodology is useful for tracking the spread of resistance but does not provide information on the strength of this resistance or its impact. The concentration of insecticide used has no relationship to the quantity of insecticide used in field applications but is instead set as twice the concentration required to kill a susceptible strain of the same species." Bagi et al 2015, Pg. 2.
- 14
"In 2011 and 2012, the 50% lethality time (LT50) for the VK7 strain of An. gambiae mosquitoes was determined by varying the length of exposure time (60–600 min). The mean mortality rate was recorded per time point, and the LT50 was estimated by fitting a logistic regression model by using logit-transformed probabilities (10) in R statistical software (http://www.r-project.org)." Toé et al 2014, Pg. 1692.
- 15
"In Prof. Ranson’s opinion, researchers have relied for too long on World Health Organization (WHO)-standard bioassays. These bioassays are designed so that exposure to a given level of insecticide kills 100 percent of a susceptible mosquito population (any survivors indicate resistance), which makes them useful tools for detecting the appearance of resistance in a population. However, they don’t measure the degree of resistance.
"Recently researchers have pushed for bioassay data that is useful in predicting the impact of resistance rather than just monitoring its spread. Some (including those working on the President’s Malaria Initiative (PMI) five-country investigation of the impact of insecticide resistance on malaria vector control) have begun to use more quantitative bioassays that expose mosquitoes to increasing doses of insecticide to determine how much is needed to exterminate a given population." GiveWell's non-verbatim summary of a conversation with Hilary Ranson, Professor, Medical Entomology, Liverpool School of Tropical Medicine, March 18, 2016. - 16
- Experimental hut trials are conducted as follows: "Methods: Studies in areas where mosquitoes breed. Volunteers sleep in experimental huts for a specific period under an ITN or an UTN, with one hut per person. The huts are identical in construction, and incorporate exit traps to catch wild mosquitoes entering and exiting the hut prematurely. Each morning of the trial, both live and dead mosquitoes, blood fed and non-blood fed, are collected and counted from both inside the hut and the exit traps. Live mosquitoes are monitored for a further 24 h to assess delayed mortality. Volunteers and nets are randomly allocated to huts at the start of the trial and are usually rotated to avoid bias. Often huts are cleaned between rotations to avoid cross-contamination of huts from the different treatment arms [25]." Strode et al 2014, Pg. 2.
- "Perhaps of more direct relevance in decision making are assays that measure the response of local vectors to locally implemented vector control tools. The simplest example of this is the cone bioassay in which mosquitoes are exposed to a bed net or a sprayed wall for a fixed exposure time and then mortality recorded 1 h and 24 h after exposure [15]. Results from cone bioassays paint an alarming picture, with very low kill rates being observed even after exposure to new nets or freshly sprayed surfaces in several settings [8,11,13,16–18]." Ranson and Lissenden 2016, Pg. 3.
- 17
“The distribution of pyrethroid resistance in African malaria vectors was described in a 2011 article in this journal [3]. At this time, pyrethroid-resistant populations of Anopheles gambiae were prevalent in western and central Africa but were rarer in southern and eastern countries of the continent. Pyrethroid resistance is now widely spread across the continent with An. gambiae in Kenya, much of Tanzania, Zambia, and Zimbabwe resistant to this insecticide class. Data remain scarce for much of central Africa, although reports are emerging of pyrethroid resistance across the Democratic Republic of Congo [4]. In summary, although An. gambiae populations fully susceptible to pyrethroids are still present in 2015 [e.g., in parts of Angola, Madagascar, and Mozambique...] they are becoming increasingly outnumbered by resistant populations (Figure 1A).
"Data on resistance in Anopheles funestus remain limited (Figure 1B) but, whereas previously pyrethroid resistance in this species was thought to be restricted to southern Africa, it has now been detected in Uganda [5], Kenya [5], Benin [6], and Cameroon [7]. As with An. gambiae, fully susceptible populations of An. funestus have been reported in some areas of Mozambique (Figure 2D) but resistance is very prevalent in other areas of the country [8]. Furthermore, the trend is similar to that observed in An. gambiae, with susceptible populations becoming the exception rather than the norm.” Ranson and Lissenden 2016, Pgs. 1-2.
- 18
- ”Data are extracted monthly from peer reviewed scientific publications and other published reports including the President’s Malaria Initiative Country Insecticide Susceptibility Summaries and IRBase (a section of VectorBase). Note: some countries have no published records on insecticide resistance and hence data are not currently reported on IR Mapper." IR Mapper – Data sources – 2016.
- "IR Mapper is a joint initiative. Data collection and collation is performed by Vestergaard, data proofreading is conducted by KEMRI/CDC and the interactive map platform was developed by ESRI Eastern Africa (collectively “IR Mapper”)." IR Mapper – Data sources – 2016.
- 19
- "Alternative measures of resistance in which the intensity or strength of the resistance is measured have been described [11] and are being adopted in a small number of field studies [12–14]. Again, longitudinal monitoring of resistance intensity can provide an important insight on the rapid changes occurring in malaria vectors. For example, the exposure time required to kill 50% of the An. gambiae population in an area in southwestern Burkina Faso was found to increase tenfold over a single year [13]." Ranson and Lissenden 2016, Pg. 3.
- "The resistance levels we report in the current study are alarming. Because few studies have attempted to quantify resistance strength in field populations, it is difficult to know if this extreme resistance phenotype is exceptional or symptomatic of the status of pyrethroid resistance in malaria vectors in Africa. Two other studies have used the LT50 method to assess the strength of resistance to pyrethroids in field populations compared with susceptible (laboratory raised) strains. In 2011, deltamethrin resistance ratios of 138-fold were recorded in Tiassalé, Côte d’Ivoire (13) and 292-fold in Jinja, Uganda (16). Thus, to our knowledge, deltamethrin resistance levels of 730-fold in 2012 (estimated by LT50) and >1,000 fold in 2013 (estimated by LD50) reported in the current study are the highest in the published literature." Toé et al 2014, Pg. 1694.
- The two other studies referenced in Toé et al 2014 are Mawejje et al 2013 and Choi et al 2014.
- 20
- “In 2011 and 2012, the 50% lethality time (LT50) for the VK7 strain of An. gambiae mosquitoes was determined by varying the length of exposure time (60–600 min). The mean mortality rate was recorded per time point, and the LT50 was estimated by fitting a logistic regression model by using logit-transformed probabilities (10) in R statistical software (http://www.r-project.org).
"In 2013, CDC bottle bioassays were used to quantify the level of resistance to deltamethrin. Glass 250-mL bottles were coated with different concentration of deltamethrin ranging from 3.125 µg/mL to 125 µg/mL at CNRFP. Bottles were prepared according to CDC guidelines (11). Female mosquitoes (3–5 days) were aspirated into bottles for 1 h and subsequently transferred to insecticide-free paper cups for 24 h of observation. Four to six replicates were performed for each concentration and for the control bottles (impregnated with acetone). Equivalent age mosquitoes of the Kisumu strain were exposed to various insecticide concentrations (range 0.001 µg/mL–0.5 µg/mL). The 50% lethal dose (LD50) was determined by using R statistical software.” Toé et al 2014, Pg. 1692. - "The resistance levels we report in the current study are alarming. Because few studies have attempted to quantify resistance strength in field populations, it is difficult to know if this extreme resistance phenotype is exceptional or symptomatic of the status of pyrethroid resistance in malaria vectors in Africa. Two other studies have used the LT50 method to assess the strength of resistance to pyrethroids in field populations compared with susceptible (laboratory raised) strains. In 2011, deltamethrin resistance ratios of 138-fold were recorded in Tiassalé, Côte d’Ivoire (13) and 292-fold in Jinja, Uganda (16). Thus, to our knowledge, deltamethrin resistance levels of 730-fold in 2012 (estimated by LT50) and >1,000 fold in 2013 (estimated by LD50) reported in the current study are the highest in the published literature." Toé et al 2014, Pg. 1694.
- “In 2011 and 2012, the 50% lethality time (LT50) for the VK7 strain of An. gambiae mosquitoes was determined by varying the length of exposure time (60–600 min). The mean mortality rate was recorded per time point, and the LT50 was estimated by fitting a logistic regression model by using logit-transformed probabilities (10) in R statistical software (http://www.r-project.org).
- 21
- "The resistance levels we report in the current study are alarming. Because few studies have attempted to quantify resistance strength in field populations, it is difficult to know if this extreme resistance phenotype is exceptional or symptomatic of the status of pyrethroid resistance in malaria vectors in Africa. Two other studies have used the LT50 method to assess the strength of resistance to pyrethroids in field populations compared with susceptible (laboratory raised) strains. In 2011, deltamethrin resistance ratios of 138-fold were recorded in Tiassalé, Côte d’Ivoire (13) and 292-fold in Jinja, Uganda (16). Thus, to our knowledge, deltamethrin resistance levels of 730-fold in 2012 (estimated by LT50) and >1,000 fold in 2013 (estimated by LD50) reported in the current study are the highest in the published literature." Toé et al 2014, Pg. 1694.
- "[N]o major difference was seen in pyrethroid mortality rates over a 3-year period with a fixed exposure of insecticide. However, when exposure time or concentration was varied, increases in the strength of the resistance were observed." Toé et al 2014, Pg. 1694.
- 22
- "In Africa, An. gambiae, An. arabiensis and An. funestus are co-dominant across much of the continent, whereas in the Asian-Pacific region there is a highly complex situation with multi-species coexistence and variable species dominance." Sinka et al 2012, Pg. 1.
- On diagnostic test outcomes, see Figure 1, Ranson and Lissenden 2016, Pg. 2.
- 23
See Figure 2, Ranson and Lissenden 2016, Pg. 3.
- 24
- "Data are extracted monthly from peer reviewed scientific publications and other published reports including the President’s Malaria Initiative Country Insecticide Susceptibility Summaries and IRBase (a section of VectorBase). Note: some countries have no published records on insecticide resistance and hence data are not currently reported on IR Mapper." IR Mapper – Data sources – 2016.
- "IR Mapper is a joint initiative. Data collection and collation is performed by Vestergaard, data proofreading is conducted by KEMRI/CDC and the interactive map platform was developed by ESRI Eastern Africa (collectively “IR Mapper”)." IR Mapper – Data sources – 2016.
- 25
See, e.g.:
- "Malaria control is reliant on insecticides to control the mosquito vector. As efforts to control the disease have intensified, so has the selection pressure on mosquitoes to develop resistance to these insecticides. The distribution and strength of this resistance has increased dramatically in recent years and now threatens the success of control programs.” Ranson and Lissenden 2016, Pg. 1.
- "Pyrethroid resistance was first detected in the two major malaria vectors Anopheles gambiae s.l. and Anopheles funestus in a small number of sites at the end of the last century [2–4]. The 21st century has witnessed a rapid spread of this resistance phenotype across the continent and it is now difficult to find sites in Africa where both vectors remain fully susceptible to pyrethroids [5]," Bagi et al 2015, Pg. 2.
- 26
"Researchers lack a clear understanding of both the extent and the clinical impact of insecticide resistance. In particular, there are not any high-quality studies that enable researchers to confidently estimate the expected impact of current levels of insecticide resistance on clinical outcomes (e.g., mortality due to malaria)." GiveWell's non-verbatim summary of a conversation with Hilary Ranson, Professor, Medical Entomology, Liverpool School of Tropical Medicine, March 18, 2016
- 27
"[I]t is a recognised ethical principle that patients should receive the best proven standard of care whenever feasible." Nardini 2014, Pg. 5.
- 28
See:
- Lindblade et al 2015 (in Malawi):
- On sample size: "A fixed cohort of 1,199 children aged six to 59 months was seen monthly for one year and at sick visits to measure malaria infection and use of ITNs." Pg. 1
"Table 3, Predictors of malaria incidence in a fixed cohort of 1,199 children age six to 59 months at baseline, Liwonde, Malawi 2012- 2013 ... Characteristic Bed net use ITN 1710 UTN 92 No bed net 86," Pg. 11. - On methods and results: "Methods: One deltamethrin ITN was distributed in the study area for every two individuals in each household plus one extra ITN for households with an odd number of residents. A fixed cohort of 1,199 children aged six to 59 months was seen monthly for one year and at sick visits to measure malaria infection and use of ITNs. Insecticide resistance among malaria vectors was measured. The effect of ITN use on malaria incidence was assessed, adjusting for potential confounders using generalized estimating equations accounting for repeated measures.
"Results: There were 1,909 infections with Plasmodium falciparum over 905 person-years at risk (PYAR), resulting in an observed incidence of 2.1 infections per person-year (iPPY). ITNs were used during 97% of the PYAR. The main vector was Anopheles funestus: mortality in WHO tube assays after exposure to 0.05% deltamethrin was 38% (95% confidence interval (CI) 29–47), and resistance was due to elevated oxidase enzymes. After adjusting for potential confounders, the incidence of malaria infection among ITN users was 1.7 iPPY (95% CI 1.5-2.1) and among non-bed net users was 2.6 iPPY (95% CI 2.0-3.3). Use of ITNs reduced the incidence of malaria infection by 30% (rate ratio 0.7; 95% CI, 0.5-0.8) compared to no bed nets." Pg. 1.
- On sample size: "A fixed cohort of 1,199 children aged six to 59 months was seen monthly for one year and at sick visits to measure malaria infection and use of ITNs." Pg. 1
- Asidi et al 2012 (in Benin).
- On sample size: "Eleven households at the southern sites (where mosquitoes are resistant) and 5 households at the northern site (where mosquitoes are susceptible) participated in the study." Pg. 1102.
- On methods: "In this comparative study in areas of contrasting pyrethroid resistance and susceptibility, we used vector blood feeding as a surrogate for malaria risk and demonstrated that ITNs lose their capacity to provide protection once An. gambiae M form develops pyrethroid resistance of the type found in southern Benin (16). These findings clearly show that ITNs in local use fail to protect against An. gambiae populations that contain kdr resistance at high frequency. The mechanisms of resistance in southern Benin are complex, and metabolic resistance appears to contribute (14–16). The demonstration of contrasting blood-feeding and survival rates between resistant and susceptible An. gambiae mosquitoes in the household trial corroborates findings and predictions from earlier experimental hut studies in southern and northern Benin and confirms the veracity of experimental huts as a tool for predicting protection or control in the home (16,22)." Pg. 1104.
- Lindblade et al 2015 (in Malawi):
- 29
“Methods: One deltamethrin ITN was distributed in the study area for every two individuals in each household plus one extra ITN for households with an odd number of residents. A fixed cohort of 1,199 children aged six to 59 months was seen monthly for one year and at sick visits to measure malaria infection and use of ITNs. Insecticide resistance among malaria vectors was measured. The effect of ITN use on malaria incidence was assessed, adjusting for potential confounders using generalized estimating equations accounting for repeated measures.” Lindblade et al 2015, Pg. 1.
- 30
“The objective of this investigation was to assess the effectiveness of ITNs to prevent malaria in an area of Malawi with moderate pyrethroid resistance...Use of ITNs reduced the incidence of malaria infection by 30% (rate ratio 0.7; 95% CI, 0.5-0.8) compared to no bed nets." Lindblade et al 2015, Pg. 1.
- 31
“Results: There were 1,909 infections with Plasmodium falciparum over 905 person-years at risk (PYAR), resulting in an observed incidence of 2.1 infections per person-year (iPPY). ITNs were used during 97% of the PYAR. The main vector was Anopheles funestus: mortality in WHO tube assays after exposure to 0.05% deltamethrin was 38% (95% confidence interval (CI) 29–47), and resistance was due to elevated oxidase enzymes. After adjusting for potential confounders, the incidence of malaria infection among ITN users was 1.7 iPPY (95% CI 1.5-2.1) and among non-bed net users was 2.6 iPPY (95% CI 2.0-3.3). Use of ITNs reduced the incidence of malaria infection by 30% (rate ratio 0.7; 95% CI, 0.5-0.8) compared to no bed nets." Lindblade et al 2015, Pg. 1.
- 32
“Pyrethroid resistance is becoming widespread in Anopheles gambiae mosquitoes, coinciding with expanded use of insecticide-treated nets (ITNs) throughout Africa. To investigate whether nets in use are still protective, we conducted household trials in northern and southern Benin, where An. gambiae mosquitoes are susceptible and resistant, respectively, to pyrethroids. Rooms were fitted with window traps and monitored for mosquito biting and survival rates before and after the nets were treated with pyrethroid. Sleeping under an ITN in the location with resistant mosquitoes was no more protective than sleeping under an untreated net, regardless of its physical condition. By contrast, sleeping under an ITN in the location with susceptible mosquitoes decreased the odds of biting by 66%. ITNs provide little or no protection once the mosquitoes become resistant and the netting acquires holes. Resistance seriously threatens malaria control strategies based on ITN.” Asidi et al 2012, Abstract.
- 33
“Sleeping under an ITN in the location with resistant mosquitoes was no more protective than sleeping under an untreated net, regardless of its physical condition. By contrast, sleeping under an ITN in the location with susceptible mosquitoes decreased the odds of biting by 66%. ITNs provide little or no protection once the mosquitoes become resistant and the netting acquires holes. Resistance seriously threatens malaria control strategies based on ITN.” Asidi et al 2012, Abstract.
- 34
“Eleven households at the southern sites (where mosquitoes are resistant) and 5 households at the northern site (where mosquitoes are susceptible) participated in the study." Asidi et al 2012, Pg. 1102.
- 35
“The researchers identified 25 reports of laboratory and field studies of the impact of ITNs on African malaria vectors that measured the mosquitoes’ resistance to pyrethroid insecticides at the time of the study. The laboratory studies used two assays to measure entomological outcomes. The cone test measured mosquito mortality (death), percent of mosquitoes knocked down (immobilized) after 60 minutes, and the time to knock down 50% or 95% of the mosquitoes after brief exposure to an ITN or untreated bed net (UTN). In the tunnel test, mosquitoes had to pass through a holed ITN or UTN to reach animal baits; counts of live and dead mosquitoes, and fed and unfed mosquitoes on both sides of the net measured deterrence, blood feeding, and mosquito mortality. In the field studies, volunteers slept under an ITN or UTN in an experimental hut. Subsequent counts of live and dead mosquitoes and fed and unfed mosquitoes inside the huts and in exit traps measured deterrence, blood feeding, mosquito mortality, and induced exophily.” Strode et al 2014, Pg. 32.
- 36
- Description of experimental hut trials: "Field Trials
"Methods: Studies in areas where mosquitoes breed. Volunteers sleep in experimental huts for a specific period under an ITN or an UTN, with one hut per person. The huts are identical in construction, and incorporate exit traps to catch wild mosquitoes entering and exiting the hut prematurely. Each morning of the trial, both live and dead mosquitoes, blood fed and non-blood fed, are collected and counted from both inside the hut and the exit traps. Live mosquitoes are monitored for a further 24 h to assess delayed mortality. Volunteers and nets are randomly allocated to huts at the start of the trial and are usually rotated to avoid bias. Often huts are cleaned between rotations to avoid cross-contamination of huts from the different treatment arms [25].
"Outcomes: Deterrence, blood feeding, mosquito mortality, and induced exophily.
"Advantages: Given that this method assesses the response of wild mosquitoes to human volunteers, it is a more realistic representation of how effective ITNs are in terms of entomological outcomes, compared with laboratory methods." Strode et al 2014, Pg. 2.
- Description of the meta-analysis: "We included laboratory and field studies of African malaria vectors that measured resistance at the time of the study and used World Health Organization–recommended impregnation regimens. We reported mosquito mortality, blood feeding, induced exophily (premature exit of mosquitoes from the hut), deterrence, time to 50% or 95% knock-down, and percentage knock-down at 60 min. Publications were searched from 1 January 1980 to 31 December 2013 using MEDLINE, Cochrane Central Register of Controlled Trials, Science Citation Index Expanded, Social Sciences Citation Index, African Index Medicus, and CAB Abstracts. We stratified studies into three levels of insecticide resistance, and ITNs were compared with untreated bed nets (UTNs) using the risk difference (RD). ... Included were 36 laboratory and 24 field studies, reported in 25 records. Studies tested and reported resistance inconsistently. Based on the meta-analytic results, the difference in mosquito mortality risk for ITNs compared to UTNs was lower in higher resistance categories. However, mortality risk was significantly higher for ITNs compared to UTNs regardless of resistance. For cone tests: low resistance, risk difference (RD) 0.86 (95% CI 0.72 to 1.01); moderate resistance, RD 0.71 (95% CI 0.53 to 0.88); high resistance, RD 0.56 (95% CI 0.17 to 0.95). For tunnel tests: low resistance, RD 0.74 (95% CI 0.61 to 0.87); moderate resistance, RD 0.50 (95% CI 0.40 to 0.60); high resistance, RD 0.39 (95% CI 0.24 to 0.54). For hut studies: low resistance, RD 0.56 (95% CI 0.43 to 0.68); moderate resistance, RD 0.39 (95% CI 0.16 to 0.61); high resistance, RD 0.35 (95% CI 0.27 to 0.43). However, with the exception of the moderate resistance category for tunnel tests, there was extremely high heterogeneity across studies in each resistance category (chi-squared test, p,0.00001, I2 varied from 95% to 100%)." Strode et al 2014, Pg. 1.
- Description of experimental hut trials: "Field Trials
- 37
"This meta-analysis found that ITNs are more effective than UTNs regardless of resistance. There appears to be a relationship between resistance and the RD for mosquito mortality in laboratory and field studies. However, the substantive heterogeneity in the studies’ results and design may mask the true relationship between resistance and the RD, and the results need to be interpreted with caution." Strode et al 2014, Pg. 1.
- 38
"Studies overall are very poor in characterising the resistance pattern of the mosquitoes, and the classification systems are unclear and lack uniformity. ... We observed a large amount of heterogeneity and bias across studies, which was particularly acute in the field studies. ... In addition, the execution of the field trials was not uniform across the studies, e.g., washing of nets, rotation of nets/sleepers, season in which the trial took place, length of the trial, decontamination of huts, and exclusion of male mosquitoes from the analysis." Strode et al 2014, Pg. 28.
- 39
- Trape et al 2011 reports results as follows: "Methods We did a longitudinal study of inhabitants of Dielmo village, Senegal, between January, 2007, and December, 2010. We monitored the inhabitants for fever during this period and we treated malaria attacks with artesunate plus amodiaquine. In July, 2008, we offered longlasting insecticide (deltamethrin)-treated nets (LLINs) to all villagers. We did monthly night collections of mosquitoes during the whole study period, and we assessed asymptomatic carriage from cross-sectional surveys. Our statistical analyses were by negative binomial regression, logistic regression, and binomial or Fisher exact test.
"Findings There were 464 clinical malaria attacks attributable to Plasmodium falciparum during 17 858 person-months of follow-up. The incidence density of malaria attacks averaged 5·45 (95% CI 4·90–6·05) per 100 person-months between January, 2007, and July, 2008, before the distribution of LLINs. Incidence density decreased to 0·41 (0·29–0·55) between August, 2008, and August, 2010, but increased back to 4·57 (3·54–5·82) between September and December, 2010—ie, 27–30 months after the distribution of LLINs. The rebound of malaria attacks were highest in adults and children aged 10 years or older: 45 (63%) of 71 malaria attacks recorded in 2010 compared with 126 (33%) of 384 in 2007 and 2008 (p<0·0001). 37% of Anopheles gambiae mosquitoes were resistant to deltamethrin in 2010, and the prevalence of the Leu1014Phe kdr resistance mutation increased from 8% in 2007 to 48% in 2010 (p=0·0009).
Interpretation Increasing pyrethroid resistance of A gambiae and increasing susceptibility of older children and adults, probably due to decreasing immunity, caused the rebound and age shift of malaria morbidity. Strategies to address the problem of insecticide resistance and to mitigate its effects must be urgently defined and implemented." Pg. 1. - Maharaj, Mthembu and Sharp 2005 interpreted the spike in malaria cases in KwaZulu-Natal as follows: "Archival malaria case data show that low levels of malaria transmission have occurred in South Africa since the early 1970s. A significant increase in the number of malaria cases was detected in KwaZulu-Natal following the discontinuation of DDT use in 1996 and the discovery of a pyrethroid-resistant vector mosquito species during the 1999/2000 epidemic. With the increasing burden of malaria morbidity and on the advice of experts, the national health department took the decision to return to DDT indoor residual spraying in March 2002. The effect of spraying was immediately made evident by the rapid decline in the number of cases reported from high-risk areas treated with DDT. A further decrease in malaria incidence occurred after the mop-up spraying in May 2000. It was evident that DDT contributed significantly to this decrease in morbidity since it was the only variable to have changed at the peak of the epidemic. Further gains in malaria control were made following the introduction of artemisinin-based combination therapy in February 2001." Pg. 873.
- Trape et al 2011 reports results as follows: "Methods We did a longitudinal study of inhabitants of Dielmo village, Senegal, between January, 2007, and December, 2010. We monitored the inhabitants for fever during this period and we treated malaria attacks with artesunate plus amodiaquine. In July, 2008, we offered longlasting insecticide (deltamethrin)-treated nets (LLINs) to all villagers. We did monthly night collections of mosquitoes during the whole study period, and we assessed asymptomatic carriage from cross-sectional surveys. Our statistical analyses were by negative binomial regression, logistic regression, and binomial or Fisher exact test.
- 40
We rely on the following three observational studies:
- Louis et al 2015 found that under-5 malaria cases increased following a 2010 ITN distribution in Burkina Faso. See: "Objective To investigate if the first national insecticide-treated bed-net campaign in Burkina Faso, done in 2010, was followed by a decrease in childhood malaria in a district with high baseline transmission of the disease. ... Findings The bed-net campaign was associated with an increase in the reported use of insecticide-treated nets. In 2009, 73% (630/869) of children reportedly slept under nets. In 2011, 92% (449/487) did. The campaign had no effect on the proportion of young children with P. falciparum parasitaemia after the rainy season; 52% (442/858) in 2009 and 53% (263/499) in 2011. Cases of malaria increased markedly after the campaign, as did the number of children presenting with other diseases. The campaign was not associated with any changes in child mortality." Pg. 750.
- Jagannathan et al 2012 followed a cohort of 100 children in Tororo, Uganda from 2008 to 2011, finding that risk of malaria increased 52% even despite provision of ITNs and prompt medical treatment. See: "Methods: A cohort of 100 children, aged six weeks to 10 months of age, were enrolled in an area of high malaria transmission intensity and followed through 48 months of age. Children were given a long-lasting insecticide-treated bed net (LLIN) at enrolment and received all care, including monthly blood smears and treatment with artemisinin-based combination therapy (ACT) for uncomplicated malaria, at a dedicated clinic. The incidence of malaria was estimated by passive surveillance and associations between malaria incidence and age, calendar time and season were measured using generalized estimating equations.
"Results: Reported compliance with LLINs was 98% based on monthly routine evaluations. A total of 1,633 episodes of malaria were observed, with a median incidence of 5.3 per person-year (PPY). There were only six cases of complicated malaria, all single convulsions. Malaria incidence peaked at 6.5 PPY at 23 months of age before declining to 3.5 PPY at 48 months. After adjusting for age and season, the risk of malaria increased by 52% from 2008 to 2011 (RR 1.52, 95% CI 1.10-2.09). Asymptomatic parasitaemia was uncommon (monthly prevalence <10%) and rarely observed prior to 24 months of age.
Conclusions: In Tororo, despite provision of LLINs and prompt treatment with ACT, the incidence of malaria is very high and appears to be rising. Additional malaria control interventions in high transmission settings are likely needed." Pg. 1. - Mukonka et al 2014 show that malaria prevalence in Nchelenge District, Luapula Province, Zambia increased from 2006 to 2012 even as intervention coverage with ITNs and indoor residual spraying increased. See: "Methods: Yearly aggregated information on cases of malaria, malaria deaths, use of malaria diagnostics, and malaria control interventions from 2006 to 2012 were obtained from the Nchelenge District Health Office. Trends in the number of malaria cases, methods of diagnosis, malaria positivity rate among pregnant women, and intervention coverage were analysed using descriptive statistics.
"Results: Malaria prevalence remained high, increasing from 38% in 2006 to 53% in 2012. Increasing numbers of cases of severe malaria were reported until 2010. Intense seasonal malaria transmission was observed with seasonal declines in the number of cases between April and August, although malaria transmission continued throughout the year. Clinical diagnosis without accompanying confirmation declined from 95% in 2006 to 35% in 2012. Intervention coverage with long-lasting insecticide-treated nets and indoor residual spraying increased from 2006 to 2012." Pg. 1.
- 41
"Prof. Ranson believes bed nets are still working well in most places, but researchers have found evidence of bed net failure in some areas with high resistance." GiveWell's non-verbatim summary of a conversation with Hilary Ranson, Professor, Medical Entomology, Liverpool School of Tropical Medicine, March 18, 2016.
- 42
"Methods: Cross-sectional surveys of LLINs were conducted in houses of seven and four villages in Gem and Bungoma Districts in western Kenya, respectively. Condition (number and area of holes in the nets), number and species of mosquitoes resting inside them, and insecticidal activity of nets were quantified. Mosquitoes collected inside nets were allowed to lay eggs and progeny tested for susceptibility to deltamethrin and permethrin, pyrethoids commonly deployed in LLINs in western Kenya.
"Results: In Gem, 83.3% of nets were less than three years old and 32.4% had at least one hole of any size; while in Bungoma, 92% were less than three years old and 48% had at least one hole. No anopheline and five Culex spp. mosquitoes were found resting inside nets in Gem regardless of the number and size of holes, while 552 Anopheles gambiae s.l., five Anopheles funestus s.l. and 137 Culex spp. were in nets in Bungoma. The number of mosquitoes resting inside nets increased with hole areas >50 cm in Bungoma. In WHO resistance assays, f1 offspring of samples collected in nets in Bungoma were 94 and 65% resistant to deltamethrin and permethrin, respectively. Nets from Bungoma retained strong activity against a susceptible laboratory strain, but not against f1 offspring of field-collected An. gambiae s.s. All An. gambiae s.s. samples collected in nets were homozygous for the kdr genotype L1014S." Ochomo et al 2013, Pg. 1. - 43
Ojuka et al 2015 reports: "Background: Southwestern Uganda has high malaria heterogeneity despite moderate vector control and other interventions. Moreover, the early biting transmission and increased resistance to insecticides might compromise strategies relying on vector control. ...
"Results: ... In both areas and seasons, an unusually early evening biting peak was observed between 6 - 8 p.m. In Engari, insecticide bioassays showed 85%, 34% and 12% resistance to DDT during the rainy season, dry season and to deltamethrin during the dry season, respectively. In Kigorogoro, 13% resistance to DDT and to deltamethrin was recorded. There was no resistance observed to bendiocarb and pirimiphos methyl." Pg. 1. - 44
See, e.g., Tokponnon et al 2014, which found that ITNs were more effective at reducing malaria risk in high resistance areas in South-West Benin than in low resistance areas: See: "Methods: The study was conducted in four rural districts and included 32 villages categorized as low or high resistance areas in Plateau Department, south-western Benin. Larvae collection was conducted to measure vector susceptibility to deltamethrin and knockdown resistance (kdr) frequency. In each resistance area, around 500 children were selected to measure the prevalence of malaria infection as well as the prevalence of anaemia associated with the use of LLINs.
"Results: Observed mortalities of Anopheles gambiae s.s population exposed to deltamethrin ranged from 19 to 96%. Knockdown resistance frequency was between 38 and 84%. The prevalence of malaria infection in children under five years was 22.4% (19.9-25.1). This prevalence was 17.3% (14.2-20.9) in areas of high resistance and 27.1% (23.5-31.1) in areas of low resistance (p = 0.04). Eight on ten children that were aged six - 30 months against seven on ten of those aged 31–59 months were anaemic. The anaemia observed in the six to 30-month old children was significantly higher than in the 31–59 month old children (p = 0.00) but no difference associated with resistance areas was observed (p = 0.35). The net use rate was 71%. The risk of having malaria was significantly reduced (p < 0.05) with LLIN use in both low and high resistance areas. The preventive effect of LLINs in high resistance areas was 60% (95% CI: 40–70), and was significantly higher than that observed in low resistance areas (p < 0.05)." Pg. 1. - 45
- "Prof. Ranson believes bed nets are still working well in most places, but researchers have found evidence of bed net failure in some areas with high resistance." GiveWell's non-verbatim summary of a conversation with Hilary Ranson, Professor, Medical Entomology, Liverpool School of Tropical Medicine, March 18, 2016.
- ”LLINs in good physical condition undoubtedly still provide protection against malaria and the spread of resistance should not derail plans to increase access to the most effective tool to reduce malaria transmission. However, the malaria community cannot afford to be complacent about insecticide resistance. Critical knowledge gaps on the causes and consequences of insecticide resistance need to be filled (see Outstanding Questions), the development, evaluation, and implementation of new products must be accelerated, and an evidence base for how best to deploy insecticide to minimize the spread of resistance must be generated to ensure the success of future malaria control efforts." Ranson and Lissenden 2016, Pg. 9.
- 46
"Whilst there is extensive evidence of resistance in Anopheles mosquitoes, there is little evidence of control programme failure associated directly with insecticide resistance largely because of many confounding factors." Kleinschmidt et al 2015, Pg. 2.
- 47
We have not seen any models which seem to account for all of the relevant factors that we can think of (e.g., ITN usage, temperature, endemicity of malaria, etc.) using methods that we understand and feel confident are sound. We do not know whether the modeling studies discussed on this page take into account factors such as whether insecticide resistance may disrupt herd immunity.
- 48
Experimental hut trials are conducted as follows: "Methods: Studies in areas where mosquitoes breed. Volunteers sleep in experimental huts for a specific period under an ITN or an UTN, with one hut per person. The huts are identical in construction, and incorporate exit traps to catch wild mosquitoes entering and exiting the hut prematurely. Each morning of the trial, both live and dead mosquitoes, blood fed and non-blood fed, are collected and counted from both inside the hut and the exit traps. Live mosquitoes are monitored for a further 24 h to assess delayed mortality. Volunteers and nets are randomly allocated to huts at the start of the trial and are usually rotated to avoid bias. Often huts are cleaned between rotations to avoid cross-contamination of huts from the different treatment arms [25]." Strode et al 2014, Pg. 2.
- 49
Briet et al 2013 parameterizes 9 data sources from 2007 - 2010 (see "Table 1 Data sources," Pg. 2), whereas the unpublished paper parameterizes 46 data sources from 2008 - 2015.
- 50
- Following Strode et al 2014, we define “moderate resistance” as a mosquito mortality rate of about 25-80% in a WHO bioassay, or an average mortality rate of roughly 55%. See Strode et al 2014, Table 4, Pg. 5.
- AMF’s largest distribution to date is in Uganda, where it is presently working to support the distribution of about 10.7 million ITNs. The following graph (provided by AMF) collates the percentage mortality in all published WHO bioassays testing for resistance to pyrethroids in Uganda:
Based on this graph, we estimate that the average prevalence of insecticide resistance (as measured in WHO bioassays) in areas where AMF will distribute nets was ~55% in 2013. We would expect this rate to fall over time and to be worse in 2016-2017 (when the bulk of AMF nets are expected to be distributed), but on the other hand, these measures of insecticide resistance may have been taken in areas where researchers thought they would be more likely to find resistance, biasing the estimate toward lower mosquito mortality rates. We note that we have not vetted the studies underlying the graph, or whether it is a comprehensive account of all bioassay studies in Uganda.
- "AMF has signed an agreement with Uganda’s Ministry of Health to fund 10.7 million long-lasting insecticidal nets (LLINs) for distribution in Uganda’s upcoming universal coverage campaign (UCC). ... Key elements of our agreement include: AMF is funding 10.7m LLINs (US$26,438,253.60)," Against Malaria Foundation - AMF funds 10.7 million nets for distribution in Uganda.
- "Tab 2: Insecticide resistance (Considered in assessing appropriate districts and sub-districts for distribution of PBO nets) - A list and summary of all Insecticide resistance (IR) studies in Uganda," AMF - Uganda Distributions Spreadsheet.
- Figure 1 from Ranson and Lissenden 2016 (Pgs. 1-2) shows an average of a ~65% mosquito mortality rate in 2014 for WHO bioassays collected across sub-Saharan Africa. This figure would likely be lower in 2016-2017 but also may be substantially biased toward a lower mortality rate than the actual average mosquito mortality rate if researchers are more likely to collect WHO bioassays in areas where they expect to find high levels of insecticide resistance.
- 51
- Given a mosquito mortality rate of 55%, the model estimates that where the dominant vector is Anopheles gambiae and there is 80% LLIN coverage, we should expect an absolute increase in clinical malaria cases of ~200 per 1,000 people per year. For a fully susceptible population, the model predicts ~200 clinical cases of malaria per 1,000 people per year. For a completely resistant population, it predicts ~800 clinical cases of malaria per 1,000 people per year. Thus, the model predicts that instead of ITNs averting 600 malaria cases per 1,000 people, they avert only 400, a one-third drop in efficacy.
- Note that Anopheles gambiae is commonly a dominant vector for malaria in sub-Saharan Africa. For example, it is the dominant vector in Uganda. "Anopheles gambiae s.s is the dominant vector species and shows a sympatric distribution with An. funestus and less abundant An. arabiensis." National Malaria Control Programme Uganda - An epidemiological profile of malaria and its control in Uganda, Pg. 13.
- 52
Some sanity checks that we considered include:
- At a high level, it seems that we should expect insecticide resistance to reduce the effectiveness of bed nets by somewhere between 5% and 80%. The existence of any detectable insecticide resistance suggests that we should expect nets to at least have a small but meaningful reduction in effectiveness (i.e., at least about 5%). On the other hand, we estimate that about 20% of the impact of bed nets is attributable to the physical barrier of the net alone (i.e., without insecticide), implying a worst case scenario of an 80% reduction in effectiveness due to insecticide resistance. See sheet “Bednets-IR” in our cost-effectiveness analysis for details.
- Informally, it seems that insecticide resistance is a major concern but that most malaria experts believe that nets remain highly effective. Roughly translating this into a quantiative estimate might imply a discount in the 15-50% range.
- An extremely simplistic model of insecticide resistance could assume a linear relationship between mosquito mortality and the effectiveness of bed nets. If 80% of bed nets’ effectiveness is due to insecticide rather than the physical barrier, and the insecticide is now 30% less effective than it was (i.e., assuming a mosquito mortality rate of about 70%), this would imply a reduction in the effectiveness of bed nets of about 24% (80%*30%=24%).
- Previously, we had arbitrarily assumed that insecticide resistance reduced nets’ effectiveness by about 20% (see the 2015 version of our cost-effectiveness analysis on this page). Since recent evidence seems to imply that insecticide resistance is worsening over time, it seems that we should adjust our cost-effectiveness analysis accordingly.
- 53
- On pyrethroid and pyriproxyfen (PPF) nets see, e.g., "Conclusion: Olyset Duo was superior to Olyset Net in terms of personal protection and killing of pyrethroid resistant An. gambiae, and sterilized surviving blood-fed mosquitoes. Mixing pyrethroid and pyriproxyfen on a LN shows potential for malaria control and management of pyrethroid resistant vectors by preventing further selection of pyrethroid resistant phenotypes." Ngufor et al 2014, Pg. 1.
- On pyrethroid and piperonyl butoxide (PBO) nets see, e.g., "Methods: A multi centre experimental hut trial was conducted in Malanville (Benin), Vallée du Kou (Burkina Faso) and Pitoa (Cameroon) to investigate the exophily, blood feeding inhibition and mortality induced by PermaNet® 3.0 (i.e. a mosaic net containing piperonyl butoxide and deltamethrin on the roof) comparatively to the WHO recommended PermaNet® 2.0 (unwashed and washed 20-times) and a conventionally deltamethrin-treated net (CTN).
Results: The personal protection and insecticidal activity of PermaNet 3.0 and PermaNet® 2.0 were excellent (>80%) in the “pyrethroid-tolerant” area of Malanville. In the pyrethroid-resistance areas of Pitoa (metabolic resistance) and Vallée du Kou (presence of the L1014F kdr mutation), PermaNet® 3.0 showed equal or better performances than PermaNet® 2.0. It should be noted however that the deltamethrin content on PermaNet® 3.0 was up to twice higher than that of PermaNet® 2.0. Significant reduction of efficacy of both LLIN was noted after 20 washes although PermaNet® 3.0 still fulfilled the WHO requirement for LLIN." Corbel et al 2010, Pg. 1.
- 54
"Methods: A 2 armed cluster-randomised controlled trial will be conducted in Burkina Faso to assess whether PPF-LLIN (containing 2% permethrin and 1% pyriproxyfen w/w) provide better protection against clinical malaria in children than 2% permethrin-treated LLINs. Study subjects will be recruited and provided with LLINs at the start of the study. The LLINs will be exchanged for PPF-LLIN by cluster in a step-wedge fashion so 3 months before the end of the 2 year trial all participants will have a PPF-LLIN. The primary endpoint will be clinical malaria incidence measured by passive case detection in a cohort of children, aged 6 months to 5 years. Anaemia and parasite prevalence will also be measured in children during cross-sectional surveys. Exposure to malaria parasites will be assessed using light traps followed by identification of common vector species and their sporozoite infection rates. Safety evaluation will include recording of adverse events and pregnancy outcomes. The main endpoint analysis will include adjusting for distance between village clusters with different types of nets, as the impact of PPF-LLIN is likely to increase as larger areas are dominated by PPF-LLIN, reducing the spill over of mosquitoes from villages with LLINs." Tiono et al 2015, Pg. 1.
- 55
"Assuming prevalence of P. falciparum around 30% [37], 20 clusters in each arm and CV = 0.3, the study could detect a 30% reduction of prevalence, with 5% significance and 80% power, between the study arms if longitudinal cohorts for each cluster contain at least 45 children. Assuming mean Hb around 10.5g/dL, 20 clusters in each arm and CV = 0.1, the study could detect a difference of 1.0g/dL in mean Hb, with 5% significance and 80% power, between the study arms if at least 35 children were followed in each cluster." Tiono et al 2015, Pg. 9.
- 56
- "The proposed study is a four-arm randomized control trial (RCT) in 48 villages in the Lakes region in Tanzania comparing the relative effectiveness of 4 vector control interventions for reducing malaria transmission and controlling vector populations in an area where An gambiae s.s is pyrethroid and carbamate resistant: 1/ a standard long lasting insecticidal net (LLIN), 2/ a LLIN which incorporates a piperonyl butoxide (PBO) synergist, 3/ a long lasting indoor residual spray (IRS) formulation used in conjunction with standard pyrethroid LLIN or 4/ the long lasting indoor residual spray (IRS) formulation used in conjunction with the LLIN which incorporates a PBO synergist."
- "Intervention Other: Olyset Plus LLIN Other: Conventional Olyset LLIN Other: IRS"
ClinicalTrials.gov registration of PBO net study.
- 57
"Estimated Enrollment: 3840; Study Start Date: September 2014; Estimated Study Completion Date: January 2017," ClinicalTrials.gov registration of PBO net study.
- 58
"Background: Long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) are the main malaria prevention interventions in Ethiopia. ... Methods/Design: This 2 x 2 factorial cluster randomized controlled trial is being carried out in the Adami Tullu district in south-central Ethiopia for about 116 weeks from September 2014 to December 2016. The trial is based on four arms: LLINs + IRS, LLINs alone, IRS alone and control. Villages (or clusters) will be the unit of randomization. The sample size includes 44 clusters per arm, with each cluster comprised of approximately 35 households (about 175 people). Prior to intervention, all households in the LLINs + IRS and LLINs alone arms will be provided with LLINs free of charge. Households in the LLINs + IRS and IRS alone arms will be sprayed with carbamate propoxur once a year just before the main malaria transmission season throughout the investigation. The primary outcome of this trial will be a malaria incidence based on the results of the rapid diagnostic tests in patients with a fever or history of fever attending health posts by passive case detection. Community-based surveys will be conducted each year to assess anemia among children 5–59 months old. In addition, community-based malaria prevalence surveys will be conducted each year on a representative sample of households during the main transmission season. The cost-effectiveness of the interventions and entomological studies will be simultaneously conducted. Analysis will be based on an intention-to-treat principle." Deressa et al 2016, Pg. 1.
- 59
- "A multi-country study was undertaken in Sudan, Kenya, India, Cameroon and Benin to quantify the potential loss of epidemiological effectiveness of ITNs and IRS due to decreased susceptibility of malaria vectors to insecticides." Kleinschmidt et al 2015, Pg. 1
- "Table 2: Study implementation details by country" Kleinschmidt et al 2015, Pg. 7.
- 60
- "Methods: Malaria disease incidence rates by active case detection in cohorts of children, and indicators of insecticide resistance in local vectors were monitored in each of approximately 300 separate locations (clusters) with high coverage of malaria vector control over multiple malaria seasons. Phenotypic and genotypic resistance was assessed annually. In two countries, Sudan and India, clusters were randomly assigned to receive universal coverage of ITNs only, or universal coverage of ITNs combined with high coverage of IRS." Kleinschmidt et al 2015, Pg. 1.
- "Measuring IR in many locations is also challenging and requires standardization of procedures and testing conditions such as temperature and humidity. There are more sensitive methods of resistance determination such as generation of population specific concentration or lethal exposure time curves [24, 38]. However these better metrics of the intensity of IR require multiple exposures per cluster which was not possible in logistically constrained study settings. The WHO susceptibility test measuring mosquito mortality within 24 h after 60 min of exposure to a standard concentration of insecticide on impregnated papers is commonly used and was considered operationally the most feasible." Kleinschmidt et al 2015, Pg. 12.
- 61
"In Sudan, Kenya, Cameroon and Benin data collection is due to be completed in 2015. In India data collection will be completed in 2016." Kleinschmidt et al 2015, Pg. 1.
- 62
“Comparison was made to a WHOPES-recommended LN treated with permethrin alone (Olyset Net; Sumitomo Chemical Company Ltd) and a LN treated with pyriproxyfen alone, which was formulated to the same technical specifications as Olyset Duo.” Ngufor et al 2014, Pg. 3.
- 63
"Olyset Plus nets (with PBO) are made of yarn with a smaller mesh size compared to Olyset nets." GiveWell's non-verbatim summary of a conversation with Hilary Ranson, Professor, Medical Entomology, Liverpool School of Tropical Medicine, March 18, 2016.
